Abstract
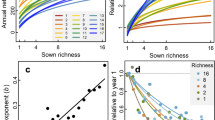
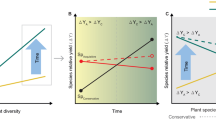
The dynamic equilibrium model of species diversity predicts that ecosystem productivity interacts with disturbance to determine how many species coexist. However, a robust test of this model requires manipulations of productivity and disturbance over a sufficient timescale to allow competitive exclusion, and such long-term experimental tests of this hypothesis are rare. Here we use long-term (27 years), large-scale (8 × 50-m plots), factorial manipulations of soil resource availability and sheep grazing intensity (disturbance) in grasslands to test the dynamic equilibrium model. As predicted by the model, increased productivity not only reduced plant species richness, but also moderated the effects of grazing intensity, shifting them from negative to neutral with increasing productivity. Reductions in species richness with productivity were associated with dominance by faster growing (i.e. high specific leaf area) and taller plants. Conversely, grazing favoured shorter plants and this effect became stronger with greater productivity, consistent with the view that grazing can lead to weaker asymmetric competition for light. Our study shows that the dynamic equilibrium model can help to explain changes in plant species richness following long-term increases in soil resource availability and grazing pressure, two fundamental drivers of change in grasslands worldwide.


Similar content being viewed by others
References
Adler PB, Seabloom EW, Borer ET, Hillebrand H, Hautier Y et al (2011) Productivity is a poor predictor of plant species richness. Science 333:1750–1753. doi:10.1126/science.1204498
Aerts R (1999) Interspecific competition in natural plant communities: mechanisms, trade-offs and plant-soil feedbacks. J Exp Bot 50:29–37
Aiken LS, West SG (1991) Multiple regression: testing and interpreting interactions. Sage, Newbury Park
Asner GP, Elmore AJ, Olander LP, Martin RE, Harris AT (2004) Grazing systems, ecosystem responses, and global change. Annu Rev Environ Resour 29:261–299
Bagchi S, Ritchie ME (2010) Herbivore effects on above- and belowground plant production and soil nitrogen availability in the Trans-Himalayan shrub-steppes. Oecologia 164:1075–1082. doi:10.1007/s00442-010-1690-5
Bakker ES, Ritchie ME, Olff H, Milchunas DG, Knops JMH (2006) Herbivore impact on grassland plant diversity depends on habitat productivity and herbivore size. Ecol Lett 9:780–788. doi:10.1111/j.1461-0248.2006.00925.x
Balvanera P, Pfisterer AB, Buchmann N, He J-S, Nakashizuka T, Raffaelli D, Schmid B (2006) Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol Lett 9:1146–1156
Bates D, Maechler M (2010) lme4: linear mixed-effects models using S4 classes. The Comprehensive R Archive Network (CRAN), Vienna
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bouwman AF, Van der Hoek KW, Eickhout B, Soenario I (2005) Exploring changes in world ruminant production systems. Agric Syst 84:121–153
Connell J (1971) On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In: Den Boer PJ, Gradwell GR (eds) Dynamics of populations. PUDOC, Wageningen
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199:1302–1310
Coulter JD (1975) The climate. In: Kuschel G (ed) Biogeography and ecology in New Zealand. Junk, The Hague, pp 87–138
Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, Helkowski JH, Holloway T, Howard EA, Kucharik CJ, Monfreda C, Patz JA, Prentice IC, Ramankutty N, Snyder PK (2005) Global consequences of land use. Science 309:570–574
Frank DA (2005) The interactive effects of grazing ungulates and aboveground production on grassland diversity. Oecologia 143:629–634. doi:10.1007/s00442-005-0019-2
Garnier E, Shipley B, Roumet C, Laurent G (2001) A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct Ecol 15:688–695
Garnier E, Cortez J, Billès G, Navas M-L, Roumet C, Debussche M, Laurent G, Blanchard A, Aubry D, Bellmann A, Neill C, Toussaint J-P (2004) Plant functional markers capture ecosystem properties during secondary succession. Ecology 85:2630–2637
Garnier E, Lavorel S, Ansquer P, Castro H, Cruz P, Dolezal J, Eriksson O, Fortunel C, Freitas H, Golodets C, Grigulis K, Jouany C, Kazakou E, Kigel J, Kleyer M, Lehsten V, Leps J, Meier T, Pakeman R, Papadimitriou M, Papanastasis VP, Quested H, Quétier F, Robson M, Roumet C, Rusch G, Skarpe C, Sternberg M, Theau J-P, Thebault A, Vile D, Zarovali MP (2007) Assessing the effects of land-use change on plant traits, communities and ecosystem functioning in grasslands: a standardized methodology and lessons from an application to 11 European sites. Ann Bot 99:967–985
Gaudet CL, Keddy PA (1988) A comparative approach to predicting competitive ability from plant traits. Nature 334:242–243. doi:10.1038/334242a0
Grace JB (2001) The roles of community biomass and species pools in the regulation of plant diversity. Oikos 92:193–207. doi:10.1034/j.1600-0706.2001.920201.x
Grace JB, Michael Anderson T, Smith MD, Seabloom E, Andelman SJ, Meche G, Weiher E, Allain LK, Jutila H, Sankaran M, Knops J, Ritchie M, Willig MR (2007) Does species diversity limit productivity in natural grassland communities? Ecol Lett 10:680–689
Grime JP (1973) Competitive exclusion in herbaceous vegetation. Nature 242:344–347
Grime JP (1979) Plant strategies and vegetation processes. Wiley, Chichester
Guisan A, Edwards TC Jr, Hastie T (2002) Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Model 157:89–100. doi:10.1016/S0304-3800(02)00204-1
Haddad NM, Holyoak M, Mata TM, Davies KF, Melbourne BA, Preston K (2008) Species’ traits predict the effects of disturbance and productivity on diversity. Ecol Lett 11:348–356. doi:10.1111/j.1461-0248.2007.01149.x
Hewitt AE (1998) New Zealand soil classification. Manaaki Whenua, Lincoln
Huston MA (1979) A general hypothesis of species diversity. Am Nat 113:81–101
Huston MA (1980) Patterns of species diversity in an old field ecosystem. Bull Ecol Soc Am 61:110
Huston MA (1994) Biological diversity. Cambridge University Press, Cambridge
Huston MA (1999) Local processes and regional patterns: appropriate scales for understanding variation in the diversity of plants and animals. Oikos 86:393–401
Huston MA, McBride AC (2002) Evaluating the relative strengths of biotic versus abiotic controls on ecosystem processes. In: Loreau M, Naeem S, Inchausti P (eds) Biodiversity and ecosystem functioning: synthesis and perspectives. Oxford University Press, UK, pp 47–60
Hutchinson GE (1961) The paradox of the plankton. Am Nat 95:137–145
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528
Kneitel JM, Chase JM (2004) Disturbance, predator, and resource interactions alter container community composition. Ecology 85:2088–2093. doi:10.1890/03-3172
Laliberté E, Tylianakis JM (2012) Cascading effects of long-term land-use changes on plant traits and ecosystem functioning. Ecology 93:145–155. doi:10.1890/11-0338.1
Laliberté E, Norton DA, Tylianakis JM, Scott D (2010) Comparison of two sampling methods for quantifying changes in vegetation community structure under rangeland development. Rang Ecol Manage 63:537–545
Laliberté E, Shipley B, Norton DA, Scott D (2012) Which plant traits determine abundance under long-term shifts in soil resource availability and grazing intensity? J Ecol 100:662–677. doi:10.1111/j.1365-2745.2011.01947.x
Lambers H, Poorter H (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Adv Ecol Res 23:187–261
Lauenroth WK (2000) Methods of estimating belowground net primary production. In: Sala OE, Jackson RB, Mooney HA, Howarth RW (eds) Methods in ecosystem science. Springer, New York, pp 58–71
McGlone MS (2001) The origin of native grasslands of southeastern South Island in relation to pre-human woody ecosystems. NZ J Ecol 25:1–15
McKane RB, Johnson LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, Fry B, Giblin AE, Kielland K, Kwiatkowski BL, Laundre JA, Murray G (2002) Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415:68–71. doi:10.1038/415068a
McNaughton SJ, Oesterheld M, Frank DA, Williams KJ (1989) Ecosystem-level patterns of primary productivity and herbivory in terrestrial habitats. Nature 341:142–144
Mittelbach GG, Steiner CF, Scheiner SM, Gross KL, Reynolds HL, Waide RB, Willig MR, Dodson SI, Gough L (2001) What is the observed relationship between species richness and productivity? Ecology 82:2381–2396
Murphy WM, Silman JP, Barreto ADM (1995) A comparison of quadrat, capacitance meter, HFRO sward stick, and rising plate for estimating herbage mass in a smooth-stalked, meadowgrass-dominant white clover sward. Grass Forage Sci 50:452–455
Olff H, Ritchie ME (1998) Effects of herbivores on grassland plant diversity. Trends Ecol Evol 13:261–265
Orme CDL, Davies RG, Burgess M, Eigenbrod F, Pickup N, Olson VA, Webster AJ, Ding T-S, Rasmussen PC, Ridgely RS, Stattersfield AJ, Bennett PM, Blackburn TM, Gaston KJ, Owens IPF (2005) Global hotspots of species richness are not congruent with endemism or threat. Nature 1016–1019
Osem Y, Perevolotsky A, Kigel J (2002) Grazing effect on diversity of annual plant communities in a semi-arid rangeland: interactions with small-scale spatial and temporal variation in primary productivity. J Ecol 90:936–946
Palmer M (1994) Variation in species richness: towards a unification of hypotheses. Folia Geobot 29:511–530
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Pinheiro J, Bates D, DebRoy S (2010) nlme: linear and nonlinear mixed effects models. The Comprehensive R Archive Network (CRAN), Vienna
Proulx M, Mazumder A (1998) Reversal of grazing impact on plant species richness in nutrient-poor vs. nutrient-rich ecosystems. Ecology 79:2581–2592
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rashit E, Bazin M (1987) Environmental fluctuations, productivity, and species diversity: an experimental study. Microb Ecol 14:101–112. doi:10.1007/BF02013016
Ricklefs RE (1977) Environmental heterogeneity and plant species diversity: a hypothesis. Am Nat 111:376–381
Scholes L, Warren PH, Beckerman AP (2005) The combined effects of energy and disturbance on species richness in protist microcosms. Ecol Lett 8:730–738. doi:10.1111/j.1461-0248.2005.00777.x
Scott D (1999) Sustainability of New Zealand high-country pastures under contrasting development inputs. 1. Site, and shoot nutrients. NZ J Agric Res 42:365–383
Silvertown J (2004) Plant coexistence and the niche. Trends Ecol Evol 19:605–611. doi:10.1016/j.tree.2004.09.003
Stevens MHH, Carson WP (1999) Plant density determines species richness along an experimental fertility gradient. Ecology 80:455–465
Svensson JR, Lindegarth M, Siccha M, Lenz M, Molis M, Wahl M, Pavia H (2007) Maximum species richness at intermediate frequencies of disturbance: consistency among levels of productivity. Ecology 88:830–838. doi:10.1890/06-0976
Svensson JR, Lindegarth M, Pavia H (2010) Physical and biological disturbances interact differently with productivity: effects on floral and faunal richness. Ecology 91:3069–3080. doi:10.1890/09-0671.1
Tilman D (1982) Resource competition and community structure. Princeton University Press, Princeton
Turner BL (2008) Resource partitioning for soil phosphorus: a hypothesis. J Ecol 96:698–702
Warner RR, Chesson PL (1985) Coexistence mediated by recruitment fluctuations: a field guide to the storage effect. Am Nat 125:769–787
Westoby M (1998) A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199:213–227. doi:10.1023/A:1004327224729
Westoby M (1999) The LHS strategy in relation to grazing and fire. In: Eldridge D, Freudenberger D (eds) Proceedings VI International Rangeland Congress. International Rangeland Congress, Townsville, pp 893–896
Whittaker RH, Levin SA, Root RB (1973) Niche, habitat, and ecotope. Am Nat 107:321–338
Widdicombe S, Austen MC (2001) The interaction between physical disturbance and organic enrichment: an important element in structuring benthic communities. Limnol Oceanogr 46:1720–1733
Wilson SD, Tilman D (2002) Quadratic variation in old-field species richness along gradients of disturbance and nitrogen. Ecology 83:492–504
Worm B, Lotze HK, Hillebrand H, Sommer U (2002) Consumer versus resource control of species diversity and ecosystem functioning. Nature 417:848–851. doi:10.1038/nature00830
Zobel M (1997) The relative role of species pools in determining plant species richness: an alternative explanation of species coexistence? Trends Ecol Evol 12:266–269. doi:10.1016/S0169-5347(97)01096-3
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We thank D. Scott for allowing the use of his experiment. H. O. Venterink and R. A. Standish provided insightful comments on previous versions of this paper. P. Fortier, D. Scott, J. Morgenroth, A. Williams, G. Pilon, K. Bott, J. H. Lapointe, K. Pellerin, K. Rondeau, J. Rondeau, and E. Razavy Toosi kindly helped with field work. D. Scott, L. Kirk, A. Leckie, and N. Pink provided academic and logistical support. A. Simpson provided stock. Financial support came from the Miss E. L. Hellaby Indigenous Grassland Research Trust. E. L. was supported by the University of Canterbury, Fonds québécois de recherche sur la nature et les technologies, Education New Zealand, the University of Western Australia and the Australian Research Council (DE120100352). The experiments complied with the current laws of the country in which they were performed (New Zealand).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bernhard Schmid.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Laliberté, E., Lambers, H., Norton, D.A. et al. A long-term experimental test of the dynamic equilibrium model of species diversity. Oecologia 171, 439–448 (2013). https://doi.org/10.1007/s00442-012-2417-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2417-6