Abstract
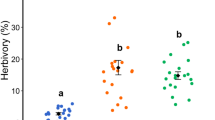
The effects of herbivory on plant fitness are integrated over a plant’s lifetime, mediated by ontogenetic changes in plant defense, tolerance, and herbivore pressure. In symbiotic ant–plant mutualisms, plants provide nesting space and food for ants, and ants defend plants against herbivores. The benefit to the plant of sustaining the growth of symbiotic ant colonies depends on whether defense by the growing ant colony outpaces the plant’s growth in defendable area and associated herbivore pressure. These relationships were investigated in the symbiotic mutualism between Cordia alliodora trees and Azteca pittieri ants in a Mexican tropical dry forest. As ant colonies grew, worker production remained constant relative to ant-colony size. As trees grew, leaf production increased relative to tree size. Moreover, larger trees hosted lower densities of ants, suggesting that ant-colony growth did not keep pace with tree growth. On leaves with ants experimentally excluded, herbivory per unit leaf area increased exponentially with tree size, indicating that larger trees experienced higher herbivore pressure per leaf area than smaller trees. Even with ant defense, herbivory increased with tree size. Therefore, although larger trees had larger ant colonies, ant density was lower in larger trees, and the ant colonies did not provide sufficient defense to compensate for the higher herbivore pressure in larger trees. These results suggest that in this system the tree can decrease herbivory by promoting ant-colony growth, i.e., sustaining space and food investment in ants, as long as the tree continues to grow.




Similar content being viewed by others
References
Barton KE, Koricheva J (2010) The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. Am Nat 175:481–493
Berenbaum MR (1995) Turnabout is fair play—secondary roles for primary compounds. J Chem Ecol 21:925–940
Boege K, Marquis RJ (2005) Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends Ecol Evol 20:441–448
Bronstein JL (1998) The contribution of ant plant protection studies to our understanding of mutualism. Biotropica 30:150–161
Bullock SH (1986) Climate of Chamela, Jalisco, and trends in the south coastal region of Mexico. Arch Meteorol Geophys Bioclimatol Ser B Theor Appl Climatol 36:297–316
Bullock SH, Solís-Magallanes JA (1990) Phenology of canopy trees of a tropical deciduous forest in Mexico. Biotropica 22:22–35
Burnham KP, Anderson DR (2010) Model selection and multimodel inference, 2nd edn. Springer, New York
Campos RI, Vasconcelos HL, Ribeiro SP, Neves FS, Soares JP (2006) Relationship between tree size and insect assemblages associated with Anadenanthera macrocarpa. Ecography 29:442–450
Cole TG, Ewel JJ (2006) Allometric equations for four valuable tropical tree species. For Ecol Manag 229:351–360
Coley PD, Kursar TA (1996) Anti-herbivore defenses of young tropical leaves: physiological constraints and ecological tradeoffs. In: Mulkey SS, Chazdon R, Smith AP (eds) Tropical forest plant ecophysiology. Chapman and Hall, New York, pp 305–336
Coomes DA, Allen RB (2007) Effects of size, competition and altitude on tree growth. J Ecol 95:1084–1097
Defossez E, Djieto-Lordon C, McKey D, Selosse MA, Blatrix R (2011) Plant-ants feed their host plant, but above all a fungal symbiont to recycle nitrogen. Proc R Soc Lond B 278:1419–1426
Del Val E, Dirzo R (2003) Does ontogeny cause changes in the defensive strategies of the myrmecophyte Cecropia peltata? Plant Ecol 169:35–41
Dirzo R, Domínguez CA (1995) Plant-herbivore interactions in Mesoamerican tropical dry forests. In: Bullock SH, Mooney HA, Medina E (eds) Seasonally dry tropical forests. Cambridge University Press, Cambridge, pp 304–325
Duarte-Rocha CF, Godoy-Bergallo H (1992) Bigger ant colonies reduce herbivory and herbivore residence time on leaves of an ant–plant: Azteca muelleri vs. Coelomera ruficornis on Cecropia pachystachya. Oecologia 91:249–252
Fischer RC, Wanek W, Richter A, Mayer V (2003) Do ants feed plants? A 15N labeling study of nitrogen fluxes from ants to plants in the mutualism of Pheidole and Piper. J Ecol 91:126–134
Fonseca CR (1993) Nesting space limits colony size of the plant-ant Pseudomyrmex concolor. Oikos 67:473–482
Frederickson ME, Gordon DM (2009) The intertwined population biology of two Amazonian myrmecophytes and their symbiotic ants. Ecology 90:1595–1607
Gordon DM (1987) Group-level dynamics in harvester ants—young colonies and the role of patrolling. Anim Behav 35:833–843
Gordon DM (1992) How colony growth affects forager intrusion between neighboring harvester ant colonies. Behav Ecol Sociobiol 31:417–427
Guedes RNC, Zanuncio TV, Zanuncio JC, Medeiros AGB (2000) Species richness and fluctuation of defoliator Lepidoptera populations in Brazilian plantations of Eucalyptus grandis as affected by plant age and weather factors. For Ecol Manag 137:179–184
Haggar JP, Ewel JJ (1997) Primary productivity and resource partitioning in model tropical ecosystems. Ecology 78:1211–1221
Harper JL (1989) The value of a leaf. Oecologia 80:53–58
Hazlett DL (1989) Provenance, age, and defoliation effects on the growth of Cordia alliodora in Central America. For Ecol Manag 28:191–202
Heil M, McKey D (2003) Protective ant–plant interactions as model systems in ecological and evolutionary research. Annu Rev Ecol Evol Syst 34:425–453
Heil M, Feil D, Hilpert A, Linsenmair KE (2004) Spatiotemporal patterns in indirect defence of a South-East Asian ant–plant support the optimal defence hypothesis. J Trop Ecol 20:573–580
Heil M, Gonzalez-Teuber M, Clement LW, Kautz S, Verhaagh M, Silva-Bueno JC (2009) Divergent investment strategies of Acacia myrmecophytes and the coexistence of mutualists and exploiters. Proc Natl Acad Sci USA 106:18091–18096
Hummel S (2000) Height, diameter and crown dimensions of Cordia alliodora associated with tree density. For Ecol Manag 127:31–40
Janzen DH (1973) Evolution of polygynous obligate acacia-ants in Western Mexico. J Anim Ecol 42:727–750
Kaspari M (2005) Global energy gradients and size in colonial organisms: worker mass and worker number in ant colonies. Proc Natl Acad Sci USA 102:5079–5083
Landis RM, Peart DR (2005) Early performance predicts canopy attainment across life histories in subalpine forest trees. Ecology 86:63–72
Lawton JH (1983) Plant architecture and the diversity of phytophagous insects. Annu Rev Entomol 28:23–39
Llandres AL, Rodríguez-Gironés MA, Dirzo R (2010) Plant stages with biotic, indirect defences are more palatable and suffer less herbivory than their undefended counterparts. Biol J Linn Soc 101:536–543
Menalled FD, Kelty MJ, Ewel JJ (1998) Canopy development in tropical tree plantations: a comparison of species mixtures and monocultures. For Ecol Manag 104:249–263
Ness JH, Morris WF, Bronstein JL (2006) Integrating quality and quantity of mutualistic service to contrast ant species protecting Ferocactus wislizeni. Ecology 87:912–921
Nomura M, Itioka T, Murase K (2001) Non-ant antiherbivore defenses before plant-ant colonization in Macaranga myrmecophytes. Popul Ecol 43:207–212
Oliver CD, Larson BC (1990) Forest stand dynamics. McGraw-Hill, New York
Olson ME, Aguirre-Hernández R, Rosell JA (2009) Universal foliage-stem scaling across environments and species in dicot trees: plasticity, biomechanics and Corner’s Rules. Ecol Lett 12:210–219
Orivel J et al (2011) Dynamics of the association between a long-lived understory myrmecophyte and its specific associated ants. Oecologia 165:369–376
Palmer TM et al (2010) Synergy of multiple partners, including freeloaders, increases host fitness in a multispecies mutualism. Proc Natl Acad Sci USA 107:17234–17239
Potvin C, Dutilleul P (2009) Neighborhood effects and size-asymmetric competition in a tree plantation varying in diversity. Ecology 90:321–327
Pringle EG et al (2011a) Distinct leaf-trait syndromes of evergreen and deciduous trees in a seasonally dry tropical forest. Biotropica 43:299–308
Pringle EG, Dirzo R, Gordon DM (2011b) Indirect benefits of symbiotic coccoids for an ant-defended myrmecophytic tree. Ecology 91:37–46
R Development Core Team R (2008) R: a language and environment for statistical computing, version 2.8.1. In. R Foundation for Statistical Computing, Vienna, Austria
SAS Institute (2009) JMP, version 8.0.2. SAS Institute, Cary, NC, USA
Smith CR, Tschinkel WR (2006) The sociometry and sociogenesis of reproduction in the Florida harvester ant Pogonomyrmex badius. J Insect Sci 6:32
Solano PJ, Dejean A (2004) Ant-fed plants: comparison between three geophytic myrmecophytes. Biol J Linn Soc 83:433–439
Stowe KA, Marquis RJ, Hochwender CG, Simms EL (2000) The evolutionary ecology of tolerance to consumer damage. Annu Rev Ecol Syst 31:565–595
Thomas SC, Sztaba AJ, Smith SM (2010) Herbivory patterns in mature sugar maple: variation with vertical canopy strata and tree ontogeny. Ecol Entomol 35:1–8
Tillberg CV (2004) Friend or foe? A behavioral and stable isotopic investigation of an ant–plant symbiosis. Oecologia 140:506–515
Trager MD, Bruna EM (2006) Effects of plant age, experimental nutrient addition and ant occupancy on herbivory in a neotropical myrmecophyte. J Ecol 94:1156–1163
Tschinkel WR (1993) Sociometry and sociogenesis of colonies of the fire ant Solenopsis invicta during one annual cycle. Ecol Monogr 63:425–457
Tschinkel WR (1999) Sociometry and sociogenesis of colony-level attributes of the Florida harvester ant (Hymenoptera: Formicidae). Ann Entomol Soc Am 92:80–89
Wagner D, Gordon DM (1999) Colony age, neighborhood density and reproductive potential in harvester ants. Oecologia 119:175–182
Warner PJ, Cushman JH (2002) Influence of herbivores on a perennial plant: variation with life history stage and herbivore species. Oecologia 132:77–85
Weiner J (2004) Allocation, plasticity and allometry in plants. Perspect Plant Ecol Evol Syst 6:207–215
Weyl EG, Frederickson ME, Yu DW, Pierce NE (2010) Economic contract theory tests models of mutualism. Proc Natl Acad Sci USA 107:15712–15716
Wheeler WM (1942) Studies of neotropical ant–plants and their ants. Bull Mus Comp Zool 90:3–262
Wulff JL (1985) Clonal organisms and the evolution of mutualism. In: Jackson JBC, Buss LW, Cook RE (eds) Population biology and evolution in clonal organisms. Yale University Press, New Haven, pp 437–466
Acknowledgments
Thanks to C. Brooks, E. Slessarev, A. Loggins, and K. Tanabe for help in the field. Megan Frederickson, Santiago Ramírez, Posy Busby, and two anonymous reviewers provided comments on the manuscript. Roger Guevara provided statistical insight. We are grateful to the Estación de Biología Chamela, UNAM, for permission to conduct the research and logistical support. This work was funded by a National Science Foundation Pre-Doctoral Fellowship and a Hubert Shaw and Sandra Lui Stanford Graduate Fellowship to E.G.P., by the Field Studies Program at Stanford University, and by a grant from the National Science Foundation (DEB # 0918848) to D.M.G. and R.D.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by John Silander.
Rights and permissions
About this article
Cite this article
Pringle, E.G., Dirzo, R. & Gordon, D.M. Plant defense, herbivory, and the growth of Cordia alliodora trees and their symbiotic Azteca ant colonies. Oecologia 170, 677–685 (2012). https://doi.org/10.1007/s00442-012-2340-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-012-2340-x