Abstract
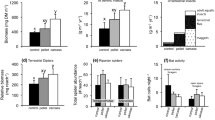
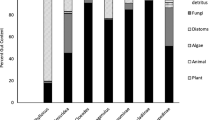
Because nutrient enrichment can increase ecosystem productivity, it may enhance resource flows to adjacent ecosystems as organisms cross ecosystem boundaries and subsidize predators in recipient ecosystems. Here, we quantified the biomass and abundance of aquatic emergence and terrestrial spiders in a reference and treatment stream that had been continuously enriched with nitrogen and phosphorus for 5 years. Because we previously showed that enrichment increased secondary production of stream consumers, we predicted that aquatic emergence flux would be higher in the treatment stream, subsequently increasing the biomass and abundance of terrestrial spiders. Those increases were predicted to be greatest for spiders specializing on aquatic emergence subsidies (e.g., Tetragnathidae). By adding a 15N stable isotope tracer to both streams, we also quantified nitrogen flow from the stream into the riparian community. Emergence biomass, but not abundance, was higher in the treatment stream. The average body size of emerging adult insects and the relative dominance of Trichoptera adults were also greater in the treatment stream. However, spider biomass did not differ between streams. Spiders also exhibited substantially lower reliance on aquatic emergence nitrogen in the treatment stream. This reduced reliance likely resulted from shifts in the body size distributions and community composition of insect emergence that may have altered predator consumption efficiency in the treatment stream. Despite nutrient enrichment approximately doubling stream productivity and associated cross-ecosystem resource flows, the response of terrestrial predators depended more on the resource subsidy’s characteristics that affected the predator’s ability to capitalize on such increases.





Similar content being viewed by others
References
Abrams PA (1993) Effect of increased productivity on the abundances of trophic levels. Am Nat 141:351–371
Alexander RB, Smith RA (2006) Trends in the nutrient enrichment of US rivers during the late 20th century and their relation to changes in probable stream trophic conditions. Limnol Oceanogr 51:639–654
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington D.C
Baxter CV, Fausch KD, Murakami M, Chapman PL (2004) Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology 85:2656–2663
Baxter CV, Fausch KD, Saunders WC (2005) Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zone. Freshwat Biol 50:201–220
Benke AC (1993) Concepts and patterns of invertebrate production in running waters. Verh Internat Verein Limnol 25:15–38
Clarke K, Gorley R (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Cross WF, Wallace JB, Rosemond AD, Eggert SL (2006) Whole-system nutrient enrichment increases secondary production in a detrital-based ecosystem. Ecology 87:1556–1565
Cross WF, Wallace JB, Rosemond AD (2007) Nutrient enrichment reduces constraints on material flows in a detritus-based food web. Ecology 88:2563–2575
Davis JM (2009) Food web response to long-term experimental enrichment of a detritus-based stream ecosystem. PhD dissertation, University of Georgia, Athens
Davis JM, Rosemond AD, Eggert SL, Cross WF, Wallace JB (2010a) Long-term nutrient enrichment decouples predator and prey production. Proc Natl Acad Sci USA 107:121–126
Davis JM, Rosemond AD, Eggert SL, Cross WF, Wallace JB (2010b) Nutrient enrichment differentially affects body sizes of primary consumers and predators in a detritus-based stream. Limnol Oceanogr 55:2305–2316
Francis TB, Schindler DE, Moore JW (2006) Aquatic insects play a minor role in dispersing salmon-derived nutrients into riparian forests in southwestern Alaska. Can J Fish Aquat Sci 63:2543–2552
Fukui D, Murakami M, Nakano S, Aoi T (2006) Effect of emergent aquatic insects on bat foraging in a riparian forest. J Anim Ecol 75:1252–1258
Gratton C, Donaldson J, vander Zanden MJ (2008) Ecosystem linkages between lakes and the surrounding terrestrial landscape in northeast Iceland. Ecosystems 11:764–774
Griffith MB, Barrows EM, Perry SA (1998) Lateral dispersal of adult aquatic insects (Plecoptera, Trichoptera) following emergence from headwater streams in forested Appalachian catchments. Ann Entomol Soc Am 91:195–201
Hall RO, Peterson BJ, Meyer JL (1998) Testing a nitrogen-cycling model of a forest stream by using a nitrogen-15 tracer addition. Ecosystems 1:283–298
Henschel JR, Mahsberg D, Stumpf H (2001) Allochthonous aquatic insects increase predation and decrease herbivory in river shore food webs. Oikos 93:429–438
Hurlbert SH (1984) Pseudreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Hynes HBN (1975) The stream and its valley. Verh Internat Verein Limnol 19:1–15
Jackson JK, Fisher SG (1986) Secondary production, emergence, and export of aquatic insects of a sonoran desert stream. Ecology 67:629–638
Kato C, Iwata T, Nakano S, Kishi D (2003) Dynamics of aquatic insect flux affects distribution of riparian web-building spiders. Oikos 103:113–120
Kato C, Iwata T, Wada E (2004) Prey use by web-building spiders: stable isotope analyses of trophic flow at a forest-stream ecotone. Ecol Res 19:633–643
Lugthart GJ, Wallace JB (1992) Effects of disturbance on benthic functional structure and production in mountain streams. J North Am Benthol Soc 11:138–164
MacNeale KH, Peckarsksy BL, Likens GE (2005) Stable isotopes identify dispersal patterns of stonefly populations living along stream corridors. Freshwat Biol 50:1117–1130
Marczak LB, Richardson JS (2007) Spiders and subsidies: results from the riparian zone of a coastal temperate rainforest. J Anim Ecol 76:687–694
Marczak LB, Hoover TM, Richardson JS (2007a) Trophic interception: how a boundary-foraging organism influences cross-ecosystem fluxes. Oikos 116:1651–1662
Marczak LB, Thompson RM, Richardson JS (2007b) Meta-analysis: trophic level, habitat, and productivity shape the food web effects of resource subsidies. Ecology 88:140–148
Moulder BC, Reichle DE (1972) Significance of spider predation in energy dynamics of forest floor arthropod communities. Ecol Monogr 42:473–498
Nakano S, Murakami M (2001) Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc Natl Acad Sci USA 98:166–170
Nentwig W, Wissel C (1986) A comparison of prey lengths among spiders. Oecologia 68:595–600
Olive CW (1980) Foraging specialization in orb-weaving spiders. Ecology 61:1133–1144
Pace ML, Cole JJ, Carpenter SR, Kitchell JF, Hodgson JR, Van de Bogert MC, Bade DL, Kritzberg ES, Bastviken D (2004) Whole-lake carbon-13 additions reveal terrestrial support of aquatic food webs. Nature 427:240–243
Paetzold A, Tockner K (2005) Effects of riparian arthropod predation on the biomass and abundance of aquatic insect emergence. J North Am Benthol Soc 24:395–402
Peterson I, Winterbottom JH, Orton S, Friberg N, Hildrew AG, Spiers DC, Gurney WSC (1999) Emergence and lateral dispersal of adult Plecoptera and Trichoptera from Broadstone Stream, UK. Freshwat Biol 42:401–416
Phillips DL, Gregg JW (2003) Source partitioning using stable isotopes: coping with too many sources. Oecologia 136:261–269
Polis GA, Hurd SD (1995) Extraordinarily high spider densities on islands: flow of energy from the marine to terrestrial food webs and the absence of predation. Proc Natl Acad Sci USA 92:4382–4386
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28:289–316
Power ME, Rainey WE, Parker MS, Sabo JL, Smyth A, Khandwala S, Finlay JC, McNeely FC, Marsee K, Anderson C (2004) River-to-watershed subsidies in an old-growth conifer forest. In: Polis GA, Power ME, Huxel GR (eds) Food webs at the landscape level. University of Chicago, Chicago, pp 217–240
Sanzone DM, Meyer JL, Marti E, Gardiner EP, Tank JL, Grimm NB (2003) Carbon and nitrogen transfer from a desert stream to riparian predators. Oecologia 134:238–250
Slavik J, Peterson BJ, Deegan LA, Bowden WB, Hershey AE, Hobbie JE (2004) Long-term responses of the Kuparuk River ecosystem to phosphorus fertilization. Ecology 85:939–954
Smith CM (2011) Biochemical plant defenses against herbivores. In: Dubinsky Z, Seckbach J (eds) All flesh is grass: plant-animal interrelationships. Springer, Netherlands, pp 287–310
Tanaka K (1991) Food consumption and diet composition of the web-building spider Agelena limbata in two habitats. Oecologia 86:8–15
Ubick D, Paquin P, Cushing PE, Roth V (eds) (2005) Spiders of North America: an identification manual. American Arachnological Society, USA
Verhoeven KJF, Simonsen KL, McIntyre LM (2005) Implementing false discovery rate control: increasing your power. Oikos 108:643–647
Wallace JB, Eggert SL, Meyer JL, Webster JR (1997) Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277:102–104
Werneke U, Zwick P (1992) Mortality of the terrestrial adult and aquatic nymphal life stages of Baetis vernus and Baetis rhodani in the Breitenbach, Germany (Insecta: Ephemeroptera). Freshwat Biol 28:249–255
Williams DD, Ambrose LG, Browning LN (1995) Trophic dynamics of two sympatric species of riparian spider (Araneae, Tetragnathidae). Can J Zool 73:1545–1553
Witman JD, Ellis JC, Anderson WB (2004) The influence of physical processes, organisms, and permeability on cross-ecosystem fluxes. In: Polis GA, Power ME, Huxel GR (eds) Food webs at the landscape level. University of Chicago Press, Chicago, pp 335–358
Acknowledgments
We appreciate field assistance from S. Dye, C. Anderson, C. Tant, and N. Taylor and advice on the experiment from J.B. Wallace, M. Bradford, P. Mulholland, F. Coyle, and A. Fisk. T. Maddox at the University of Georgia (UGA) Analytical Chemistry Lab assisted in isotopic analyses. R. Hall gave guidance on the experimental design and in developing the mixing model. Staff at the Coweeta Hydrologic Laboratory provided logistical support. K. Catley at Western Carolina University verified spider identifications. Comments from S. Dye, A. Mehring, C. Tant, D. Batzer, C. Baxter, S. Collins, L. Marczak, L. Barmuta, and an anonymous reviewer improved the manuscript. Contributions from our collaborators and funding from National Science Foundation (NSF) (DEB-9806610, DEB-0318063, DEB-9629268, and DEB-0212315) made the nutrient enrichment part of this study possible. J. Davis was funded by a NSF Graduate Research Fellowship, UGA Presidential Fellowship, and North American Benthological Society Presidential Award. During final preparation of the manuscript, J. Davis was supported by NSF Idaho EPSCoR (EPS-0814387).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Leon Barmuta.
J.M. Davis conceived the experiment, designed and conducted data collection, and analyzed the data. A.D. Rosemond contributed to experimental design. G.E. Small developed the dynamic mixing model. All authors contributed to the writing of the manuscript.
Rights and permissions
About this article
Cite this article
Davis, J.M., Rosemond, A.D. & Small, G.E. Increasing donor ecosystem productivity decreases terrestrial consumer reliance on a stream resource subsidy. Oecologia 167, 821–834 (2011). https://doi.org/10.1007/s00442-011-2026-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-011-2026-9