Abstract
Plants encounter a broad range of natural enemies and defend themselves in diverse ways. The cost of defense can be reduced if a plant secondary metabolite confers resistance to multiple herbivores. However, there are few examples of positively correlated defenses in plants against herbivores of different types. We present evidence that a genetically variable chemical trait that acts as a strong antifeedant to mammalian herbivores of Eucalyptus also deters insect herbivores, suggesting a possible mechanism for cross-resistance. We provide field confirmation that sideroxylonal, an important antifeedant for mammalian herbivores, also determines patterns of damage by Christmas beetles, a specialist insect herbivore of Eucalyptus. In a genetic progeny trial of Eucalyptus tricarpa, we found significant heritabilities of sideroxylonal concentration (0.60), overall insect damage (0.34), and growth traits (0.30–0.53). Population of origin also had a strong effect on each trait. Negative phenotypic correlations were observed between sideroxylonal and damage, and between damage and growth. No relationship was observed between sideroxylonal concentration and any growth trait. Our results suggest that potential for evolution by natural selection of sideroxylonal concentrations is not strongly constrained by growth costs and that both growth and defense traits can be successfully incorporated into breeding programs for plantation trees.
Similar content being viewed by others
Introduction
Plants have evolved a broad range of defenses against both mammalian and invertebrate herbivores. If a plant secondary metabolite (PSM) confers resistance to multiple herbivores, then the cost of defense can be reduced. However, there are few examples of positively correlated defenses in plants against herbivores of different types, such as generalists and specialists (Leimu and Koricheva 2006) or mammalian and insect herbivores (Rousi et al. 1997; Mutikainen et al. 2002; Puustinen et al. 2004). Because the challenges of multiple herbivores can place constraints on the evolution of plant defenses (Rausher 1996), the chemical and genetic basis of cross-resistance is important.
Formylated phloroglucinol compounds (FPCs) may be responsible for cross-resistance to mammalian and insect herbivores of eucalypts (Eucalyptus l’Héritier and Corymbia Hill and Johnson, Myrtaceae). These compounds are important determinants of palatability to a range of specialist and generalist mammalian herbivores, both captive and free-ranging (Lawler et al. 1998, 2000; Wallis et al. 2002; Moore et al. 2004b; Scrivener et al. 2004; Moore and Foley 2005). The physiological mechanism underlying aversion to FPCs has also been elucidated, making the system one of the best understood of plant–mammal interactions (Lawler et al. 1998). A subgroup of FPCs, known as sideroxylonals, also affects the feeding of insect herbivores in the laboratory (Floyd and Foley 2001), but evidence of deterrence in wild insects is needed to support the hypothesis that FPCs contribute to cross-resistance to mammalian and insect herbivores. We present here the first field demonstration that concentrations of sideroxylonals are related to food plant selection by wild insects, in this case a species of Christmas beetle (Anoplognathus montanus Macleay; Coleoptera: Scarabaeidae), which is a specialist herbivore of eucalypts.
There is substantial variation in the concentrations of FPCs among eucalypt trees within populations (Lawler et al. 2000; Wallis et al. 2002; Moore et al. 2004b). This can create significant habitat patchiness at ecologically relevant scales (Andrew et al. 2007), and understanding how this variation arises requires comprehension of the heritability of the trait. Narrow-sense heritability, the proportion of variation within populations that is due to additive genetic causes, is of crucial importance because it determines the ability of a species to evolve in response to both natural and artificial selection. The heritability of foliar sideroxylonal concentration has been estimated in a natural population of Eucalyptus melliodora using a marker-based method (Andrew et al. 2005), but comparisons have not yet been made with traditional common-garden estimates. Since genetic relatedness is not inferred from molecular markers and sample sizes are less likely to be limited by expense, genetic trial experiments in a common environment (often called common-garden experiments) can offer greater precision in estimates of quantitative genetic parameters. Such estimates from genetic trial experiments are more appropriate than those from natural populations for predicting likely gains from breeding programs, which are aimed at genetic improvement of useful traits in tree plantations. Common-garden experiments also allow variation among populations to be studied without the confounding effects of site differences (O’Reilly-Wapstra et al. 2002; Moore et al. 2004b). Phenotypic and genetic correlations measured in progeny can also provide insight into the evolutionary constraints on sideroxylonal concentrations.
We addressed the relationship between foliar sideroxylonal concentrations and insect herbivory and studied the quantitative genetics of sideroxylonal variation in Eucalyptus tricarpa (LAS Johnson) LAS Johnson and K.D. Hill. This was, until recently, a subspecies of E. sideroxylon (Hill and Johnson 1991), in which mammal feeding studies have been conducted (Lawler et al. 2000). The two species are chemically similar, with 0–23 mg g−1 DW sideroxylonal (mean 10.9 mg g−1 DW) in E. sideroxylon (Lawler et al. 2000; Floyd and Foley 2001) and 0–47 mg g−1 DW sideroxylonal (mean 13.5 mg g−1 DW) in E. tricarpa (this study). Since they also share mammalian and insect herbivores, the influence of foliar defenses on herbivory is likely to be sufficiently similar to provide a reasonable grounds for inferring cross-resistance. Studying a field trial testing open-pollinated progenies from a range of natural provenances, we asked the following questions:
-
1.
Do Christmas beetles preferentially attack E. tricarpa trees that have low foliar concentrations of sideroxylonal?
-
2.
Does significant narrow-sense heritability of variation in foliar sideroxylonal occur in E. tricarpa? Are heritability estimates similar to marker-based estimates from a wild E. melliodora population?
-
3.
Do E. tricarpa populations display genetic differentiation in foliar sideroxylonal concentrations?
-
4.
Do phenotypic and/or genetic correlations occur among foliar sideroxylonal, growth traits, and insect damage?
Materials and methods
Experimental design and sampling
Eucalyptus tricarpa is a widespread species occurring in the low to medium rainfall zone in Victoria and New South Wales (NSW), Australia (Brooker and Kleinig 1999). This species is closely related to E. melliodora, in which sideroxylonal is highly heritable (Andrew et al. 2005). E. tricarpa is being developed for forestry in areas of low rainfall. The E. tricarpa provenance/progeny trial sampled in this study was set up by Forests NSW, near Culcairn, NSW (146°56′E, 35°43′S) in August 2000, as part of the Australian Low Rainfall Tree Improvement Group (ALRTIG) program (Harwood et al. 2001). The trial tested 108 open-pollinated families, representing 15 natural populations in Victoria and NSW (Table 1). Eucalypts have mixed mating systems, with typical outcrossing rates of 70% (Eldridge et al. 1993). Open pollination leads to families consisting of mixtures of half-siblings and selfed or outcrossed full siblings. Trees were arranged in four replicates, with each family represented in each replicate by one five-tree row plot. Trees were planted 1.8 m apart with 4 m between rows and a buffer two trees deep on the outside edges of the trial. The experimental area covered 1.8 ha, and an adjoining 0.6-ha area was planted with surplus stock of some of the families. It was surrounded on all sides by pasture, with the nearest Eucalyptus trees approximately 200 m away.
Our sampling was conducted in November 2002, when the plants were producing foliage that was intermediate between juvenile and fully mature morphologies. The transition from juvenile to adult foliage is not as dramatic in E. tricarpa as in many other species of Eucalyptus. Leaf shape differs only slightly and foliar chemistry is stable across years (R. L. Andrew, unpublished observations). We selected 9 populations covering the natural distribution of the species and sampled from 3 to 9 families from each population (9 families where possible), giving a total of 67 families selected. In each block, we sampled the two westernmost trees of each plot planted for the selected families. Where trees were stunted or dead, the next suitable tree in the plot was sampled. From the southern side of each tree, approximately 50 g of leaf material was sampled, frozen, and stored at −20°C. We sampled one replicate at a time, so that time of day was not confounded with population or family. Height, diameter at 1.3 m (DBH) of largest stem, and the sum of the squared diameters at 1.3 m of all stems (D 2) were measured for all trees by Forests NSW in January 2004.
Insect damage
At the time of sampling, the experimental plants were under attack by Anoplognathus montanus, a local, native Christmas beetle. A simple presence/absence score was used to assess Christmas beetle attack, as the pattern of damage and beetle presence was very clear: a tree was either heavily infested or free from damage. This is consistent with previously observed patterns of damage by Anoplognathus spp. (Edwards et al. 1993). Paired comparisons were made to test the relationship between beetle infestation and sideroxylonal concentrations. Each pair consisted of one infested tree and one unaffected tree from the same family and replicate (i.e., the same plot), and sideroxylonal was measured using high-performance liquid chromatography (HPLC) for both trees (see below). The 23 pairs sampled were from 16 families, 12 of which were among those included in the quantitative genetic analysis.
In addition to Christmas beetles, defoliation and damage to photosynthetic leaf area by other agents was also apparent. A range of folivorous and sap-sucking insects, such as chrysomelid leaf beetles (Paropsis spp.) and their larvae, sawfly larvae (Perga sp.), leaf-blister sawflies (Phylacteophaga sp.), and Eucalyptus weevils (Gonipterus scutellatus), may have contributed to the observed damage. Each tree that was sampled for chemical analysis was given a score for overall insect damage. Trees were scored from one to four for minimal defoliation, light defoliation, moderate defoliation, and heavy defoliation, respectively. Heavy defoliation (damage score 4) resulted in visible bare branch ends over the whole of the canopy. Our damage classes were similar to those employed by Raymond (1995), but differed in the order of the ranking (from least to most damage) and in the description of overall damage (rather than of that produced by a single insect species). The damage score affected by the presence of Christmas beetle because of their contribution to overall leaf damage, but also describes variation in leaf damage from other sources, which were difficult to distinguish.
Chemical analysis
The leaves were freeze–dried and ground to pass through a 1-mm screen in a Tecator Cyclotec mill. A subset of 105 samples (including 46 paired trees selected for the Christmas beetle test) was assayed for sideroxylonal using the HPLC technique described by Wallis et al. (2003). Sideroxylonal A and sideroxylonal C are found in constant proportions in this solvent system (Moore et al. 2004a) and their concentrations were combined to give the concentration of total sideroxylonals, which will be referred to as sideroxylonal henceforth. We collected the visible and near-infrared spectra (400–2,500 nm) of all samples from dry, ground leaf following Moore et al. (2004b). Calibration equations were developed using modified partial least squares regression using WINISI II (Infrasoft International, Port Matilda, PA, USA) and following standard procedures (American Society for Testing and Materials 1995). The optimal model used the 1,100- to 2,498-nm region, standard normal variate and detrend scatter correction, and a math treatment of 1881 (i.e., first derivative treatment, with a gap width of 8 nm and smoothing over 8 data points; see American Society for Testing and Materials 1995). The model explained 97.8% of foliar sideroxylonal variation (mean 17.9 mg g−1 dm; range 0.0–47.6 mg g−1 dm) in the calibration set and had a standard error of cross-validation of 2.4 mg g−1 dm.
Statistical analysis
Statistical analyses were conducted using GenStat version 8 (VSN International Ltd, Hemel Hempstead, UK). The relationship between sideroxylonal and presence of Christmas beetle was investigated by choosing paired damaged and undamaged trees from the same plot. Beetle-damaged trees were paired with the nearest available undamaged tree within the same plot. This approach not only avoided the large number of zero Christmas beetle damage scores that would result if all trees assessed for sideroxylonal had been included but also controlled for genetic background (family) and location in the trial. The difference in foliar sideroxylonal concentration between the 23 affected trees and their paired unaffected trees was tested using a two-sided, one-sample t-test.
Estimates of narrow-sense heritability were computed for foliar sideroxylonal concentration, damage score, and the growth traits using pooled variance components estimated by restricted maximum likelihood analysis (REML). Variance components were estimated by fitting a mixed model, with replicate and population as fixed effects. Family within population was fitted as a random effect, as well as plot within replicate, to account for the remaining plot-level variance. Variance components estimated for families within populations (σ 2 f ), plots within replicates (σ 2plot ), and residual error (σ 2 e ) were summed to give σ 2 p , the total phenotypic variance within populations and replicates (Williams et al. 2002, p. 103). The use of a single pooled family variance component leads to an estimate of the average heritability among populations. Standard errors were calculated from the family and residual variance components using VFUNCTION (Payne 2005). All available trees were used for heritability estimation for each trait. All 108 families represented in the trial were included for height and DBH, which were measured for 1,772 and 1,692 trees, respectively. In contrast, only 67 families from nine populations were available for estimation of heritability for sideroxylonal and damage (465 trees).
Additive genetic variance in phenotypic traits can be estimated using the expected covariance of half-siblings and adjusted to account for the outcrossing rates, according to the common procedure used for trees with mixed mating systems (Eldridge et al. 1993; Williams et al. 2002). Within-population, narrow-sense heritability (h 2) specific to this environment was calculated using the formula,
where r is the mean relatedness within families, which is typically assumed to be 0.4 in Eucalyptus (Eldridge et al. 1993). Instead of four times the family variance, additive genetic variance is estimated as 2.5 times (or 1/0.4 times) the family variance. As with all half-sibling designs, this model assumes that inbreeding and epistasis are absent. Maternal effects may be important in maternal half-sibling families, but they are not commonly observed in Eucalyptus, based on controlled cross experiments (Lopez et al. 2003). In addition, this design cannot fully control for dominance effects, which may inflate the within-family covariance in plants with mixed mating systems.
Additive genetic correlations (subject to the same assumptions as for heritability estimates) between pairs of traits were estimated from plot means using multivariate REML in GenStat 8 and tested using likelihood ratio tests (Payne 2005). Replicate and population were fixed effects, while family and plot were random effects in the model. Standard errors of genetic correlations were estimated using a standard Taylor expansion formula, implemented in GenStat. Genetic correlations were estimated using only populations for which both growth and sideroxylonal had been measured.
The effects of geographic and climatic variables on phenotypic traits were tested using the best linear unbiased estimates (BLUEs) of population means obtained from the REML mixed model analysis. Geographic variables considered were latitude, longitude, and approximate altitude. Annual means of temperature and precipitation for each population were obtained using the WORLDCLIM climatic grids (Hijmans et al. 2005) in ArcView GIS 3.3 (Environmental Systems Research Institute, Redlands, CA, USA). Since minimum temperatures have been found to be related to foliar sideroxylonal concentrations in Eucalyptus microcorys (Moore et al. 2004b), this variable was also included. Univariate regression and Spearman’s rank correlation were used to test the relationships between geographic or climatic variables and the BLUEs for each trait. Since multiple tests were carried out, sequential Bonferroni corrections were used to adjust P values (Rice 1989).
Results
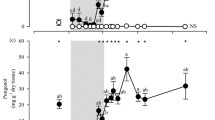
At the time of investigation, Christmas beetles occurred on 7.7% of trees sampled. The proportion of trees affected by these beetles varied from 0% to 34% among populations (Table 2), with 0–100% of trees within families under attack. Four populations were unaffected by Christmas beetles, including both NSW populations and the southernmost population, from Lorne (Fig. 1). Comparison of paired affected infested with unaffected trees showed that foliar sideroxylonal concentration was, on average, 10.4 mg g−1 dry weight lower in trees under attack (t = 3.81 on 22 df; P < 0.001).
The mean concentrations of foliar sideroxylonal in populations, predicted by the REML analysis, ranged from 12.8 to 33.8 mg g−1 dry weight (Table 2). Leaves from the Clunes, Mt Nowa Nowa and Bodalla populations were all consistently high in sideroxylonal, showing that high-sideroxylonal populations can be found throughout the range of the species. Significant differences were also observed among populations for damage score (P < 0.001), and growth traits also varied among populations (P < 0.001). Populations from Gippsland and NSW generally grew faster than those from Central and Goldfields regions (Table 2).
Genetic analysis revealed significant heritability for each trait (Table 3). Sideroxylonal and damage demonstrated heritabilities of 0.60 and 0.34, respectively. The heritabilities of height (0.44) and DBH (0.30) were high for eucalypts and higher still when estimated only for the populations in which sideroxylonal was measured (0.53 and 0.34, respectively; Table 3). Replicates differed significantly for damage and growth traits, but not for sideroxylonal concentration. The two growth traits were highly phenotypically correlated, and damage was negatively correlated with both growth and foliar sideroxylonal concentrations (Table 4). However, there was no phenotypic correlation of foliar sideroxylonal with either growth trait. The additive genetic correlation of height and DBH was high, but sideroxylonal concentration and damage score were not significantly genetically correlated with any trait (Table 4). Predicted population means were negatively correlated for insect damage and plant height (r = −0.734, P = 0.024). The correlation of predicted population means for sideroxylonal and damage score was marginally significant (r = −0.659, P = 0.054).
When the effects of source population geographic or climatic variables on traits were tested, no rank correlations and only one univariate regression was significant at the 0.05 level prior to Bonferroni corrections. However, none was significant or close to significance (P < 0.1) after corrections were made for multiple comparisons.
Discussion
Cross-resistance of insect and mammalian herbivores
This study provides field evidence that cross-resistance to insect and mammalian herbivores of Eucalyptus may be conferred by foliar sideroxylonals at typical concentrations. Trees with low concentrations of foliar sideroxylonals were chosen by Christmas beetles over nearby, genetically related trees with higher concentrations. At concentrations similar to those in the present study, sideroxylonals and other FPCs have also been shown to reduce intake by a range of mammalian herbivores (Lawler et al. 2000; Wallis et al. 2002; Moore et al. 2005). The congruence of these results with those demonstrating sideroxylonal-related resistance to herbivory by common ringtail possums (Pseudocheirus peregrinus) in a closely related and chemically similar species (E. sideroxylon, Lawler et al. 2000) suggests that cross-resistance is likely in at least these two eucalypt species, but probably also in others. The variation observed in E. tricarpa foliar sideroxylonal concentrations is most likely constitutive, as there is no evidence of induced defenses in Eucalyptus (Henery 2006). While previous tests of foliar defense induction may have failed to detect a small response, a large change in concentration would be needed to produce the 10.4-mg g−1 difference between the trees affected and unaffected by beetles in the present study.
In contrast to studies of annuals and herbaceous perennials, the chemical basis of defense has been identified in few tree-herbivore systems and can be complex (Haukioja 2003). That the same chemicals are responsible for cross-resistance to both mammalian and insect herbivores at concentrations commonly found in leaves is ecologically significant. Although broad classes of secondary chemicals, such as phenolic glycosides and terpenes, are active against both mammalian and arthropod herbivores, we are not aware of any other examples of specific chemicals known to confer resistance to both mammalian and insect herbivores. The strength of natural selection, both historical and contemporary, on FPCs in eucalypts is still unknown, but their evolution may have been favored by their effectiveness as defensive chemicals against diverse specialist and generalist herbivores.
Adult Christmas beetles are specialist herbivores of eucalypts and can cause severe damage to trees in plantations (Carne et al. 1974). They appear to have increased in numbers in rural areas, probably because their larvae feed on roots underground and can benefit from the increased productivity of pasture and crops (Carne et al. 1974). Christmas beetle numbers are extremely variable and severe outbreaks can result in nearly 100% defoliation of some trees (Pryor 1953; Edwards et al. 1993). Laboratory bioassays have shown that variation in sideroxylonal concentration in leaves can determine the feeding preferences of Christmas beetles (Floyd and Foley 2001) and this study provides evidence that wild Christmas beetles select trees on the basis of foliar sideroxylonal concentration.
In the past, Christmas beetle herbivory has been related to essential oil concentration and in particular the foliar concentration of 1,8-cineole (Edwards et al. 1993). However, resistant E. grandis clones were no higher in 1,8-cineole concentration than susceptible clones (Johns et al. 2004). Furthermore, experiments manipulating the concentration of both 1,8-cineole and sideroxylonal in leaves have suggested that FPCs have a larger effect on Christmas beetles (Floyd and Foley 2001). Foliar concentrations of cineole and sideroxylonal tend to be correlated in natural populations (Moore et al. 2004a; Andrew et al. 2005), which explains why previous studies have identified cineole as the primary deterrent. This association allows mammalian herbivores to use cineole, which is volatile and has a strong odor, as a guide to sideroxylonal concentrations (Lawler et al. 1999). It appears that terpenes may have a similar role as an olfactory cue in feeding by Christmas beetles (Floyd and Foley 2001).
In addition to resistance, plants may employ alternative or additional strategies to limit the costs of herbivory, such as tolerance (Fornoni et al. 2004) or escaping herbivory through faster growth (Kursar and Coley 2003). We found some support for the latter in E. tricarpa, as overall insect damage was related to both sideroxylonal concentration and growth. However, the relationship of damage with growth may also be due to the costs imposed by herbivore damage, since the growth measurements were made a year after the observed damage was done. A negative effect of defoliation on subsequent growth rates was observed by Raymond (1995) in trees damaged by chrysomelid leaf beetles (Chrysoptharta bimaculata), which also chose smaller trees. We were unable to separate these effects, as growth measurements were made once only and insects were not experimentally excluded.
Heritability of sideroxylonal and herbivore defense
Foliar sideroxylonal concentration is highly variable in E. tricarpa, and our estimate of the narrow-sense heritability of this trait is high. Previous progeny experiments in Eucalyptus have significant genetic differentiation in FPCs among populations, but within-population genetic variation in sideroxylonal has not yet been shown (O’Reilly-Wapstra et al. 2002, 2004). The present study supports the finding of significant additive genetic variation of foliar sideroxylonal, with narrow-sense heritability estimates between 0.55 and 0.89, in E. melliodora using a genetic marker-based approach (Andrew et al. 2005). Common-garden experiments are useful because they provide greater precision than those in natural populations. However, the coefficient of relationship of 0.4 among the individuals of open-pollinated families used for estimating heritability in this study is only an approximation (Griffin and Cotterill 1988; Eldridge et al. 1993, p. 174). The degree of relatedness within open-pollinated Eucalyptus families is affected by the levels of selfing and correlated paternity. Studies of progeny arrays using molecular markers have shown that these vary widely both among species (McDonald et al. 2003; Jones et al. 2005) and within species (Burgess et al. 1996; Butcher and Williams 2002). Direct estimates are not yet available for E. tricarpa, and the relationships among progeny within and among families may be influenced by additional factors (Squillace 1974). The true level of relatedness in field-grown progeny may therefore differ substantially from the estimate we have used, but it is clear that heritabilities of sideroxylonal concentration and insect damage in our trial are moderate to high. Our estimates of heritability, especially for growth traits, could be inflated by inbreeding depression, since the open-pollinated design does not control for differences in mating system parameters among families. The heritabilities of height and DBH are surprisingly high, as values of about 0.2 for these traits are more typical of Eucalyptus (Eldridge et al. 1993, p. 175). Estimates of heritability based on a single site may be upwardly biased due to the inclusion of a genotype × environment interaction component in the estimate of additive genetic variance (Tibbits and Hodge 1998; Costa e Silva et al. 2006). There have been few studies on chemical defenses carried out across sites (Bowers et al. 1992; Rosner and Hannrup 2004) and replicating this study at multiple sites will be a high priority for future research on FPCs.
The resistance of eucalypts to insect herbivores is often highly heritable (Raymond 1995; Jones et al. 2002; Jordan et al. 2002; Rapley et al. 2004). Insect damage was heritable in E. tricarpa and phenotypically correlated with sideroxylonal concentration, although the genetic correlation was not significant. The lack of additive genetic correlation between these traits may imply independence, and that different genes are controlling the traits or may have been due to insufficient statistical power. Genetic variation in FPC production may also explain the difference in susceptibility to Christmas beetles observed between interspecific hybrids and pure E. grandis (Shepherd 2000).
Implications for coevolution and genetic improvement
The heritability of a trait is important for understanding the evolutionary dynamics of the trait. While selection pressures are difficult to measure in long-lived trees, we now have some evidence of the evolutionary potential of foliar sideroxylonal concentrations in E. tricarpa. Our results suggest that such concentrations may be capable of responding to human-induced changes in herbivore pressures, such as increasing Christmas beetle or mammalian herbivore abundance in agricultural landscapes. As yet, it is unclear which groups of herbivores might act as the strongest selective agent on sideroxylonal, because the ability of plants to tolerate herbivory by different agents may vary (Kotanen and Rosenthal 2000). The response to breeding for increased concentrations of sideroxylonal will depend on the heritability and phenotypic variation of this trait, which our results suggest are high in E. tricarpa. Increasing leaf sideroxylonal concentration through breeding may increase the economic viability of plantation forestry by reducing the impact of insect and mammalian herbivores.
Population differentiation in heritable traits is of interest because it may result from different selection regimes, among other causes (Merila and Crnokrak 2001; McKay and Latta 2002). High foliar sideroxylonal concentrations and low variability would be expected in populations with high herbivore pressure, but other environmental factors, such as nutrient availability and rainfall, could also affect the costs and benefits of defense. Moore et al. (2004b) related differences in sideroxylonal concentrations among natural populations of E. microcorys to temperature regimes, but could not separate the effects of genetic differentiation from those of environmental differences between the sites, as experiments in controlled conditions were lacking. E. tricarpa populations displayed genetic differentiation in mean sideroxylonal concentrations, although there was no consistent pattern in differences among geographical regions, suggesting that population-level processes are important in this species, rather than broad-scale climatic variation. The lack of any strong relationship between climatic variables at the source populations and sideroxylonal or damage in this species contrasts with those present in E. microcorys (Moore et al. 2004b). This may suggest that the effects observed in the previous study may have been the result of plasticity rather than genetic differentiation driven by environmental variation, although the results of the present study are based on fewer populations and a smaller range of environmental variation. However, our results are congruent with those of Warren et al. (2005), who found only weak relationships between physiological variables and seed-source rainfall in E. tricarpa; the weak tendency of families from wetter population locations.
The long-term stability of population differences in foliar sideroxylonal concentrations is unclear. Seedling establishment and development to the sapling stage, which is likely to occur following fire in most E. tricarpa woodlands (Gill 1997), may be the stage in the life of a tree during which herbivores can inflict the most damage. Christmas beetle densities during recruitment may have a strong influence on the sideroxylonal concentrations of the next generation of E. tricarpa. The impact of Christmas beetles on eucalypts varies considerably from year to year, as populations fluctuate in response to rainfall and temperature patterns. Given the high heritability and phenotypic variance of sideroxylonal concentration, a population may respond quickly to a transient change in herbivore pressure and may not reflect the selection pressures facing the trees at present or further in the past.
Additive genetic correlations may act as evolutionary constraints, but are also important in animal and plant breeding (Lynch and Walsh 1998; Williams et al. 2002). In Eucalyptus, they could limit the potential benefits of breeding for both growth and defense. The lack of correlation between sideroxylonal and growth suggests that breeding for high sideroxylonal production need not interfere with selection gains in growth traits under plantation conditions, although genetic analysis on additional sites would be needed to draw this conclusion.
Physiological trade-offs may constrain the evolution of defense traits in plants, although evidence for the costs of plant defense has been contradictory (Koricheva 2002). No attempts have yet been made to test this hypothesis for foliar FPCs such as sideroxylonal. We found no relationship between foliar sideroxylonal concentrations and growth, implying that the physiological cost of producing sideroxylonal is not great or that the costs and benefits are balanced under the conditions of the experiment. Experiments excluding herbivores are required to measure the true physiological cost of sideroxylonal production, which may be difficult for long-lived trees with many natural enemies. Defenses have been shown to be costly in many plants, but the expression of physiological costs can depend heavily on the environmental conditions in which plants are grown (Siemens et al. 2003). Care must be taken when extrapolating from single common-garden trials such as these to natural populations.
Conclusion
We have provided field confirmation that sideroxylonal is related to resistance to an insect, as well as to mammalian herbivores. Our results support the estimate of narrow-sense heritability made for sideroxylonal using a marker-based method in a natural population of E. melliodora. We found no evidence of a strong growth-defense trade-off in E. tricarpa; however, this only highlights the need for investigation of sideroxylonal production and growth in the absence of herbivory, and of the ability of eucalypts to escape or tolerate damage by different herbivores. We have shown that differences in sideroxylonal concentration among populations have a genetic basis, although the importance of environmental factors should also be investigated. Further understanding of the inheritance of sideroxylonal could be gained from similar experiments in a range of sites aimed at detecting interactions between genotype and environment. These may be critical in applying our results to the genetic improvement of the insect resistance of eucalypts.
References
American Society for Testing and Materials (1995) Standard practices for infrared, multivariate, quantitative analysis. Designation: E1655–94. West Conshohocken, PA
Andrew RL, Peakall R, Wallis IR, Wood JT, Knight EJ, Foley WJ (2005) Can marker-based quantitative genetics in the wild be successful? Heritability and genetic correlation of chemical defences in Eucalyptus. Genetics 171:1989–1998
Andrew RL, Peakall R, Wallis IR, Foley W (2007) Spatial distribution of defense chemicals and markers: implications for the maintenance of variation. Ecology 87:716–728
Bowers MD, Collinge SK, Gamble SE, Schmitt J (1992) Effects of genotype, habitat, and seasonal variation on iridoid glycoside content of Plantago lanceolata (Plantaginaceae) and the implications for insect herbivores. Oecologia 91:201–207
Brooker MIH, Kleinig DA (1999) Field guide to eucalypts volume 1: South-Eastern Australia, 2nd edn. Bloomings Books, Hawthorn
Burgess IP, Williams ER, Bell JC, Harwood CE, Owen JV (1996) The effect of outcrossing rate on the growth of selected families of Eucalyptus grandis. Silvae Genet 45:97–100
Butcher PA, Williams ER (2002) Variation in outcrossing rates and growth in Eucalyptus camaldulensis from the Petford Region, Queensland; evidence of outbreeding depression. Silvae Genet 51:6–12
Carne PB, Greaves RTG, McInnes RS (1974) Insect damage to plantation-grown eucalypts in north coastal New South Wales, with particular reference to Christmas beetles (Coleoptera: Scarabaeidae). J Aust Entomol Soc 13:189–206
Costa e Silva J, Potts BM, Dutkowski GW (2006) Genotype by environment interaction for growth of Eucalyptus globulus in Australia. Tree Genet Genomes 2:61–75
Edwards PB, Wanjura WJ, Brown WV (1993) Selective herbivory by Christmas beetles in response to intraspecific variation in Eucalyptus terpenoids. Oecologia 95:551–557
Eldridge K, Davidson J, Harwood C, Van Wyk G (1993) Eucalypt domestication and breeding. Clarendon Press, Oxford, UK
Floyd RB, Foley WJ (2001) Identifying pest-resistant eucalypts using near-infrared spectroscopy. RIRDC Publication No 01/112. Rural Industries Research and Development Corporation, Canberra, Australia
Fornoni J, Nunez-Farfan J, Valverde PL, Rausher MD (2004) Evolution of mixed strategies of plant defense allocation against natural enemies. Evolution 58:1685–1695
Gill AM (1997) Eucalypts and fires: interdependent or independent? In: Williams J, Woinarski J (eds) Eucalypt ecology: individuals to ecosystems. Cambridge University Press, Cambridge, pp 151–167
Griffin AR, Cotterill PP (1988) Genetic variation in growth of outcrossed, selfed and open-pollinated neighbourhood inbreeding progenies of Eucalyptus regnans and some implications for breeding strategy. Silvae Genet 37:124–131
Harwood CE, Bulman P, Bush D, Mazanec R, Stackpole D (2001) Australian low rainfall tree improvement group: compendium of hardwood breeding strategies. Rural Industries Research and Development Corporation, Canberra
Haukioja E (2003) Putting the insect into the birch-insect interaction. Oecologia 136:161–168
Henery M (2006) Foliar secondary metabolites in Eucalyptus and their role in resistance to defoliating insects. PhD Thesis, The Australian National University, Canberra, Australia
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hill KD, Johnson LAS (1991) Systematic studies in eucalypts 3. New taxa and combinations in Eucalyptus (Myrtaceae). Telopea 4:223–267
Johns CV, Stone C, Hughes L (2004) Feeding preferences of the Christmas beetle Anoplognathus chloropyrus (Coleoptera: Scarabaeidae) and four paropsine species (Coleoptera: Chrysomelidae) on selected Eucalyptus grandis clonal foliage. Aust For 67:184–190
Jones TH, Potts BM, Vaillancourt RE, Davies NW (2002) Genetic resistance of Eucalyptus globulus to autumn gum moth defoliation and the role of cuticular waxes. Can J For Res 32:1961–1969
Jones RC, McKinnon GE, Potts BM, Vaillancourt RE (2005) Genetic diversity and mating system of an endangered tree Eucalyptus morrisbyi. Aust J Bot 53:11
Jordan GJ, Potts BM, Clarke AR (2002) Susceptibility of Eucalyptus globulus ssp globulus to sawfly (Perga affinis ssp insularis) attack and its potential impact on plantation productivity. For Ecol Man 160:189–199
Koricheva J (2002) Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83:176–190
Kotanen PM, Rosenthal JP (2000) Tolerating herbivory: does the plant care if the herbivore has a backbone? Evol Ecol 14:537–549
Kursar TA, Coley PD (2003) Convergence in defense syndromes of young leaves in tropical rainforests. Biochem Syst Ecol 31:929–949
Lawler IR, Foley WJ, Pass GJ, Eschler BM (1998) Administration of a 5HT3 receptor antagonist increases the intake of diets containing Eucalyptus secondary metabolites by marsupials. J Comp Physiol B 168:611–618
Lawler IR, Stapley J, Foley WJ, Eschler BM (1999) Ecological example of conditioned flavor aversion in plant–herbivore interactions: effect of terpenes of Eucalyptus leaves on feeding by common ringtail and brushtail possums. J Chem Ecol 25:401–415
Lawler IR, Foley WJ, Eschler BM (2000) Foliar concentration of a single toxin creates habitat patchiness for a marsupial folivore. Ecology 81:1327–1338
Leimu R, Koricheva J (2006) A meta-analysis of genetic correlations between plant resistances to multiple enemies. Am Nat 168:E15–E37
Lopez GA, Potts BM, Vaillancourt RE, Apiolaza LA (2003) Maternal and carryover effects on early growth of Eucalyptus globulus. Can J For Res 33:2108
Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits. Sinauer, Sunderland, Mass
McDonald MW, Rawlings M, Butcher PA, Bell JC (2003) Regional divergence and inbreeding in Eucalyptus cladocalyx (Myrtaceae). Aust J Bot 51:393–403
McKay JK, Latta RG (2002) Adaptive population divergence: markers, QTL and traits. Trends Ecol Evol 17:285–291
Merila J, Crnokrak P (2001) Comparison of genetic differentiation at marker loci and quantitative traits. J Evol Biol 14:892–903
Moore BD, Foley WJ (2005) Tree use by koalas in a chemically complex landscape. Nature 435:488–490
Moore BD, Wallis IR, Pala-Paul J, Brophy JJ, Willis RH, Foley WJ (2004a) Antiherbivore chemistry of Eucalyptus—cues and deterrents for marsupial folivores. J Chem Ecol 30:1743–1769
Moore BD, Wallis IR, Wood JT, Foley WJ (2004b) Foliar nutrition, site quality, and temperature influence foliar chemistry of tallowwood (Eucalyptus microcorys). Ecol Monogr 74:553–568
Moore BD, Foley WJ, Wallis IR, Cowling A, Handasyde KA (2005) Eucalyptus foliar chemistry explains selective feeding by koalas. Biol Lett 1:64–67
Mutikainen P, Walls M, Ovaska J, Keinänen M, Julkunen-Tiitto R, Vapaavuori E (2002) Costs of herbivore resistance in clonal saplings of Betula pendula. Oecologia 133:364–371
O’Reilly-Wapstra JM, McArthur C, Potts BM (2002) Genetic variation in resistance of Eucalyptus globulus to marsupial browsers. Oecologia 130:289–296
O’Reilly-Wapstra JM, McArthur C, Potts BM (2004) Linking plant genotype, plant defensive chemistry and mammal browsing in a Eucalyptus species. Funct Ecol 18:677–684
Payne RW (ed) (2005) The guide to genstat release 8 Part 2: statistics. VSN International, Oxford, UK
Pryor LD (1953) Variable resistance to leaf-eating insect in some eucalypts. Proc Linn Soc NSW 77:364–368
Puustinen S, Koskela T, Mutikainen P (2004) Direct and ecological costs of resistance and tolerance in the stinging nettle. Oecologia 139:76
Rapley LP, Allen GR, Potts BM (2004) Genetic variation in Eucalyptus globulus in relation to susceptibility from attack by the southern eucalypt leaf beetle, Chrysophtharta agricola. Aust J Bot 52:10
Rausher MD (1996) Genetic analysis of coevolution between plants and their natural enemies. Trends Genet 12:212–217
Raymond CA (1995) Genetic variation in Eucalyptus regnans and Eucalyptus nitens for levels of observed defoliation caused by the eucalyptus leaf-beetle, Chrysophtharta bimaculata Olivier, in Tasmania. For Ecol Man 72:21–29
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rosner S, Hannrup B (2004) Resin canal traits relevant for constitutive resistance of Norway spruce against bark beetles: environmental and genetic variability. For Ecol Man 200:77–87
Rousi M, Tahvanainen J, Henttonen H, Herms DA, Uotila I (1997) Clonal variation in susceptibility of white birches (Betula spp.) to mammalian and insect herbivores. For Sci 42:396–402
Scrivener NJ, Johnson CN, Wallis IR, Takasaki M, Foley WJ, Krockenberger AK (2004) Which trees do wild common brushtail possums (Trichosurus vulpecula) prefer? Problems and solutions in scaling laboratory findings to diet selection in the field. Evol Ecol Res 6:77–87
Shepherd M (2000) Variation and inheritance of resistance to defoliation by Christmas beetles, Anoplognathus sp. (Leach) in eucalypts. For Genet 7:57–64
Siemens DH, Lischke H, Maggiulli N, Schurch S, Roy BA (2003) Cost of resistance and tolerance under competition: the defense-stress benefit hypothesis. Evol Ecol 17:247–263
Squillace AE (1974) Average genetic correlations among offspring from open-pollinated forest trees. Silvae Genet 23:149–156
Tibbits W, Hodge G (1998) Genetic parameters and breeding value predictions for Eucalyptus nitens wood fiber production traits. For Sci 44:587–598
Wallis IR, Watson ML, Foley WJ (2002) Secondary metabolites in Eucalyptus melliodora: field distribution and laboratory feeding choices by a generalist herbivore, the common brushtail possum. Aust J Zool 50:507–519
Wallis IR, Herlt AJ, Eschler BM, Takasaki M, Foley WJ (2003) Quantification of sideroxylonals in Eucalyptus foliage by high-performance liquid chromatography. Phytochem Anal 14:360–365
Warren CR, Tausz M, Adams MA (2005) Does rainfall explain variation in leaf morphology and physiology among populations of red ironbark (Eucalyptus sideroxylon subsp tricarpa) grown in a common garden? Tree Physiol 25:1369–1378
Williams ER, Matheson AC, Harwood CE (2002) Experimental design and analysis for tree improvement, 2nd edn. CSIRO Publishing, Collingwood
Acknowledgments
The authors thank Emlyn Williams for statistical advice, and Nadine Scholtz and Junko Kondo for assistance in field and laboratory work. We are grateful to Jim and Jane Rowe and later Sharon and Lockie Altmeier for access to their property and to ALRTIG for providing background information on the trial. We also thank Ian Johnson and Hans Porada of Forests NSW for their support and assistance. We thank Brian Baltunis and anonymous reviewers for their comments on the manuscript. The work was funded by grants from the Australian Research Council and the Rural Industries Research and Development Corporation (RIRDC) to W.J.F. and by a scholarship from RIRDC and the Australian National University to R.L.A. This study complies with the laws of the country in which it was conducted, Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Julia Koricheva.
Rights and permissions
About this article
Cite this article
Andrew, R.L., Wallis, I.R., Harwood, C.E. et al. Heritable variation in the foliar secondary metabolite sideroxylonal in Eucalyptus confers cross-resistance to herbivores. Oecologia 153, 891–901 (2007). https://doi.org/10.1007/s00442-007-0784-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0784-1