Abstract
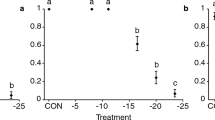
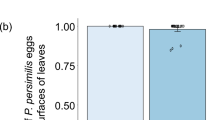
To prevent predation on their eggs, prey often avoid patches occupied by predators. As a result, they need to delay oviposition until they reach predator-free patches. Because many species allocate energy to egg production in a continuous fashion, it is not clear what kind of mechanism prey use to delay oviposition. We used females of the phytoseiid mite Neoseiulus cucumeris to study these mechanisms. Females were placed in patches with pollen, a food source they use for egg production, and they were exposed to another phytoseiid mite, Iphiseius degenerans, which is an intraguild predator of N. cucumeris juveniles. We found that the oviposition of N. cucumeris females on patches with the predator was lower than on patches without the predator. Cues left by the intraguild predator were not sufficient to elicit such behaviour. Females of N. cucumeris reduced oviposition when exposed to the predator by retaining the egg inside their body, resulting in a lower developmental rate once these eggs were laid. Hence, females are capable of retaining eggs, but the development of these eggs continues inside the mother’s body. In this way, females gain some time to search for less risky oviposition sites.



Similar content being viewed by others
References
Aljetlawi AA, Sparrevik E, Leonardsson K (2004) Prey–predator size-dependent functional response: derivation and rescaling to the real world. J Anim Ecol 73(2):239–252
Angelon KA, Petranka JW (2002) Chemicals of predatory mosquitofish (Gambusa affinis) influence selection of oviposition site by Culex mosquitoes. J Chem Ecol 28:797–805
Bell G (1980) The costs of reproduction and their consequences. Am Nat 136:20–38
Binckley CA, Resetarits WJ (2002) Reproductive decisions under threat of predation: squirrel treefrog (Hyla squirella) responses to banded sunfish (Enneacanthus obesus). Oecologia 130(1):157–161
Binckley CA, Resetarits WJ (2003) Functional equivalence of non-lethal effects: generalized fish avoidance determines distribution of gray treefrog, Hyla chrysoscelis, larvae. Oikos 102(3):623–629
Bouletreau-Merle J, Fouillet P (2002) How to overwinter and be a founder: egg-retention phenotypes and mating status in Drosophila melanogaster. Evol Ecol 16(4):309–332
Chase JM (1999) Food web effects of prey size refugia: variable interactions and alternative stable equilibria. Am Nat 154(5):559–570
De Courcy-Williams ME, Kravar-Garde L, Fenlon JS, Sunderland KD (2004) Phytoseiid mites in protected crops: the effect of humidity and food availability on egg hatch and adult life span of Iphiseius degenerans, Neoseiulus cucumeris, N. californicus, and Phytoseiulus persimilis (Acari: Phytoseiidae). Exp Appl Acarol 32:1–13
De Moraes GJ, McMurtry JA, Denmark HA, Campos CB (2004) A revised catalog of the mite family Phytoseiidae. 1st edn. Magnolia, Auckland
Eitam A, Blaustein L (2004) Oviposition habitat selection by mosquitoes in response to predator (Notonecta maculata) density. Physiol Entomol 29(2):188–191
Faraji F, Janssen A, Sabelis MW (2001) Predatory mites avoid ovipositing near counterattacking prey. Exp Appl Acarol 25:613–623
Faraji F, Janssen A, Sabelis MW (2002) Oviposition patterns in a predatory mite reduce the risk of egg predation caused by prey. Ecol Entomol 27:660–664
Janssen A, Faraji F, van der Hammen T, Magalhães S, Sabelis MW (2002) Interspecific infanticide deters predators. Ecol Lett 5:490–494
Kessler A, Baldwin IT (2002) Manduca quinquemaculata’s optimization of intra-plant oviposition to predation, food quality and thermal constraints. Ecology 83:2346–2354
Koskela E, Juutistenaho P, Mappes T, Oksanen TA (2000) Offspring defence in relation to litter size and age: experiment in the bank vole Clethrionomys glareolus. Evol Ecol 14:99–109
Magalhaes S, Janssen A, Montserrat M, Sabelis MW (2005a) Prey attack and predators defend: counterattacking prey trigger parental care in predators. Proc R Soc Lond B 272(1575):1929–1933
Magalhaes S, Tudorache C, Montserrat M, van Maanen R, Sabelis MW, Janssen A (2005b) Diet of intraguild predators affects antipredator behavior in intraguild prey. Behav Ecol 16(2):364–370
Mappes J, Kaitala A (1995) Host–plant selection and predation risk for offspring of the parent bug. Ecology 76(8):2668–2670
Mokany A, Shine R (2003) Oviposition site selection by mosquitoes is affected by cues from conspecific larvae and anuran tadpoles. Austral Ecol 28(1):33–37
Montgomerie RD, Weatherhead PJ (1988) Risks and rewards of nest defence by parent birds. Quart Rev Biol 63:167–187
Murphy PJ (2003a) Context-dependent reproductive site choice in a Neotropical frog. Behav Ecol 14(5):626–633
Murphy PJ (2003b) Does reproductive site choice in a neotropical frog mirror variable risk facing offspring? Ecol Monogr 73(1):45–67
Nomikou M, Janssen A, Sabelis MW (2003) Herbivore host plant selection: whitefly learns to avoid host plants that harbour predators of her offspring. Oecologia 136:484–488
Polis GA, Holt RD (1992) Intraguild predation—the dynamics of complex trophic interactions. Trends Ecol Evol 7:151–154
Reguera P, Gomendio M (2002) Flexible oviposition behavior in the golden egg bug (Phyllomorpha laciniata) and its implications for offspring survival. Behav Ecol 13(1):70–74
Resetarits WJ (2001) Colonization under threat of predation: avoidance of fish by an aquatic beetle, Tropisternus lateralis (Coleoptera: Hydrophilidae). Oecologia 129(1):155–160
Sabelis MW (1981) Biological control of two-spotted spider mites using phytoseiid predators. Part 1. PhD Thesis, University of Wageningen, Wageningen
Stav G, Blaustein L, Margalith J (1999) Experimental evidence for predation risk sensitive oviposition by a mosquito, Culiseta longiareolata . Ecol Entomol 24(2):202–207
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and descent of man. Aldine, Chicago, IL
Trivers RL (1974) Parent–offspring conflict. Am Zool 14:249–264
van Rijn PCJ, Tanigoshi LK (1999) Pollen as food source for the predatory mites Iphiseius degenerans and Neoseiulus cucumeris (Acari: Phytoseiidae): dietary range and life history. Exp Appl Acarol 23:785–802
Williams GC (1966) Natural selection, the cost of reproduction and the refinement of Lack’s principle. Am Nat 100:687–690
Zhang Y, Zhang ZQ, Lin J, Ji J (2000) Potential of Amblyseius cucumeris (Acari: Phytoseiidae) as a biocontrol agent against Schizotetranychus nanjingensis (Acari: Tetranychidae) in Fujian, China. Syst Appl Acarol Spec Publ 4:109–124
Acknowledgments
Maria Nomikou, Belén Belliure, Tessa van der Hammen, Pauline de Bruijn, João Ferreira, Christian Tudorache and Roos van Maanen are thanked for discussions. M. M. and A. J. were employed by the University of Amsterdam within the framework of an NWO-Pioneer project granted to A. M. de Roos, C. B. was supported by the Departament d’Universitats, Recerca i Societat de la Informació, and S. M. was supported by a Praxis XXI grant from the Portuguese government. The animals used for the research described in this publication are not test animals in the legal sense.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Sven Bacher.
Rights and permissions
About this article
Cite this article
Montserrat, M., Bas, C., Magalhães, S. et al. Predators induce egg retention in prey. Oecologia 150, 699–705 (2007). https://doi.org/10.1007/s00442-006-0527-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0527-8