Abstract
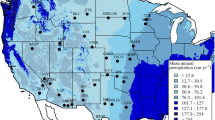
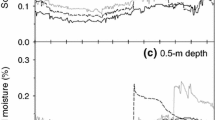
In the arid and semiarid regions of North America, discrete precipitation pulses are important triggers for biological activity. The timing and magnitude of these pulses may differentially affect the activity of plants and microbes, combining to influence the C balance of desert ecosystems. Here, we evaluate how a “pulse” of water influences physiological activity in plants, soils and ecosystems, and how characteristics, such as precipitation pulse size and frequency are important controllers of biological and physical processes in arid land ecosystems. We show that pulse size regulates C balance by determining the temporal duration of activity for different components of the biota. Microbial respiration responds to very small events, but the relationship between pulse size and duration of activity likely saturates at moderate event sizes. Photosynthetic activity of vascular plants generally increases following relatively larger pulses or a series of small pulses. In this case, the duration of physiological activity is an increasing function of pulse size up to events that are infrequent in these hydroclimatological regions. This differential responsiveness of photosynthesis and respiration results in arid ecosystems acting as immediate C sources to the atmosphere following rainfall, with subsequent periods of C accumulation should pulse size be sufficient to initiate vascular plant activity. Using the average pulse size distributions in the North American deserts, a simple modeling exercise shows that net ecosystem exchange of CO2 is sensitive to changes in the event size distribution representative of wet and dry years. An important regulator of the pulse response is initial soil and canopy conditions and the physical structuring of bare soil and beneath canopy patches on the landscape. Initial condition influences responses to pulses of varying magnitude, while bare soil/beneath canopy patches interact to introduce nonlinearity in the relationship between pulse size and soil water response. Building on this conceptual framework and developing a greater understanding of the complexities of these eco-hydrologic systems may enhance our ability to describe the ecology of desert ecosystems and their sensitivity to global change.






Similar content being viewed by others
References
Alder NN, Sperry JS, Pockman WT (1996) Root and stem xylem embolism, stomatal conductance, and leaf turgor in Acer grandidentatum populations along a soil moisture gradient. Oecologia 105:293–301
Austin AT, Yahdjian ML, Stark JM, Belnap J, Porporato A, Norton U, Ravetta DA, Schaeffer SM (2004) Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia. DOI 10.1007/s00442-004-1519-1
Baldocchi DD, Wilson KB, Gu LH (2002) How the environment, canopy structure and canopy physiological functioning influence carbon, water and energy fluxes of a temperate broad-leaved deciduous forest—an assessment with the biophysical model CANOAK. Tree Physiol 22:1065–1077
BassiriRad H, Tremmel DC, Virginia RA, Reynolds JF, de Soyza AG, Brunell MH (1999) Short-term patterns in water and nitrogen acquisition by two desert shrubs following a simulated summer rain. Plant Ecol 145:27–36
Belnap J, Phillips SL, Miller ME (2004) Response of desert biological soil crusts to alteration in precipitation frequency. Oecologia. DOI 10.1007/s00442-003-1438-6
Bergkamp G (1998) A hierarchical view of the interactions of runoff and infiltration with vegetation and microtopography in semiarid shrublands. CATENA 33:201–220
Bhark EW, Small EE (2003) Association between plant canopies and the spatial patterns of infiltration in shrubland and grassland of the Chihuahuan desert, New Mexico. Ecosystems 6:185–196
Boyer JS (1985) Water transport. Annu Rev Plant Physiol 36:473–516
Burgess SSO, Adams MA, Turner NC, Ong CK (1998) The redistribution of soil water by tree root systems. Oecologia 115:306–311
Burgess SSO, Pate JS, Adams MA, Dawson TE (2000) Seasonal water acquisition and redistribution in the Australian woody phreatophyte, Banksia prionotes. Ann Bot 85:215–224
Cable JM, Huxman TE (2004) Precipitation pulse size effects on Sonoran Desert soil microbial crusts. Oecologia. DOI 10.1007/s00442-003-1461-7
Caldwell MM, Richards JH (1989) Hydraulic lift—water efflux from upper roots improves effectiveness of water-uptake by deep roots. Oecologia 79:1–5
Chabot BF, Hicks DJ (1982) The ecology of leaf life spans. Annu Rev Ecol Syst 13:229–259
Chesson P, Gebauer RL, Schwinning S, Huntly N, Wiegand K, Ernest MSK, Sher A, Novoplansky A, Weltzin JF (2004) Resource pulses, species interactions and diversity maintenance in arid and semi-arid environments. Oecologia. DOI 10.1007/s00442-004-1551-1
Cunningham GL, Burke JH (1973) The effect of carbonate deposition layers (“caliche”) on the water status of Larrea divaricata. Am Midl Nat 90:474–480
Davidowitz G (2002) Does precipitation variability increase from mesic to xeric biomes? Global Ecol Biogeogr 11:143–154
Davis SD, Mooney HA (1985) Comparative water relations of adjacent California shrub and grassland communities. Oecologia 66:522–529
Dawson TE (1993) Hydraulic lift and water use by plants: implications for water balance, performance and plant–plant interactions. Oecologia 95:565–574
Devitt DA, Smith SD (2002) Root channel macropores enhance downward movement of water in a Mojave Desert ecosystem. J Arid Environ 50:99–108
Donovan LA, Ehleringer JR (1994) Carbon isotope discrimination, water-use efficiency, growth and mortality in a natural shrub population. Oecologia 100:347–354
Dougherty RL, Lauenroth WK, Singh JS (1996) Response of a grassland cactus to frequency and size of rainfall events in a North American shortgrass steppe. J Ecol 84:177–183
Dunkerley D (2002) Infiltration rates and soil moisture in a groved mulga community near Alice Springs, arid central Australia: evidence for complex internal rainwater redistribution in a runoff–runon landscape. J Arid Environ 51:199–219
Dunne T, Zhang W, Aubry B (1991) Effects of rainfall, vegetation, and microtopography on infiltration and runoff. Water Resour Res 27:2271–2285
Ehleringer JR (1985) Annuals and perennials of warm deserts. In: Chabot BF, Mooney HA (eds) Physiological ecology of North American plant communities. Chapman and Hall, New York, pp 162–180
Ehleringer JR, Schwinning S, Gebauer R (1999) Water-use in arid land ecosystems. In: Press MC, Scholes JD, Barker MG (eds) Plant physiological ecology. Blackwell, Edinburgh, pp 347–365
Eldridge D, Greene R (1994) Microbiotic soil crusts—a review of their roles in soil and ecological processes in the rangelands of Australia. Aust J Soil Res 32:289–415
Emmerich WE (2003) Carbon dioxide fluxes in a semiarid environment with high carbonate soils. Agric For Meteorol 116:91–102
Enquist BJ, Economo EP, Huxman TE, Allen AP, Ignace DD, Gillooly JF (2003) Scaling metabolism from organisms to ecosystems. Nature 423:639–642
Fay PA, Carlisle JD, Knapp AK, Blair JM, Collins SL (2003) Productivity responses to altered rainfall patterns in a C-4 dominated grassland. Oecologia 137:245–251
Flanagan LB, Ehleringer JR, Marshall JD (1992) Differential uptake of summer precipitation among co-occurring trees and shrubs in a pinyon-juniper woodland. Plant Cell Environ 15:831–836
Flanagan LB, Wever LA, Carlson PJ (2002) Seasonal and interannual variation in carbon dioxide exchange and carbon balance in a northern temperate grassland. Global Change Biol 8:599–615
Frank AB, Dugas WA (2001) Carbon dioxide fluxes over a northern, semiarid mixed-grass prairie. Agric For Meteorol 108:317–326
Green JM, Williams GJ III (1982) The subdominant status of Echinocereus viridiflorus and Mammillaria vivipara in the shortgrass prairie: the role of temperature and water effects on gas exchange. Oecologia 52:43–48
Gutierrez JR, Whitford WG (1987) Responses of Chihuahuan Desert herbaceous annuals to rainfall augmentation. J Arid Environ 12:127–139
Hamerlynck EP, Huxman TE, Smith SD, Nowak RS, Redar S, Loik ME, Jordan DN, Zitzer SR, Coleman JS, Seemann JR (2000) Photosynthetic responses of contrasting Mojave Desert shrub species to elevated CO2 concentration at the Nevada Desert FACE Facility. J Arid Environ 44:425–436
Hamerlynck EP, McAuliffe JR, McDonald EV, Smith SD (2002) Ecological responses of two Mojave Desert shrubs to soil horizon development and soil water dynamics. Ecology 83:768–779
Hamerlynck EP, Huxman TE, McAuliffe JR, Smith SD (2004) Carbon isotope discrimination and foliar nutrient status of Larrea tridentata (creosote bush) in contrasting Mojave Desert soils. Oecologia 138:210–215
Huxman TE, Turnipseed AA, Sparks JP, Harley PC, Monson RK (2003) Temperature as a control over ecosystem CO2 fluxes in a high-elevation, subalpine forest. Oecologia 134:537–546
Huxman TE, Smith MD, Fay PA, Knapp AK, Shaw MR, Loik ME, Smith SD, Tissue DT, Zak JC, Weltzin JF, Pockman WT, Sala OE, Haddad BM, Harte J, Koch GW, Schwinning S, Small EE, Williams DG (2004a) Convergence across biomes to a common rain-use efficiency. Nature 429:651–654
Huxman TE, Cable JM, Ignace DD, Eilts JA, English NB, Weltzin J, Williams DG (2004b) Response of net ecosystem gas exchange to a simulated precipitation pulse in a semiarid grassland: the role of native versus non-native grasses and soil texture. Oecologia
Kaiser WM (1987) Effect of water deficits on photosynthetic capacity. Physiol Plant 71:142–149
Kelly R, Burke I (1997) Heterogeneity of soil organic matter following death of individual plants in shortgrass steppe. Ecology 78:1256–1261
Kessavalou A, Doran JW, Mosier AR, Drijber RA (1998) Greenhouse gas fluxes following tillage and wetting in a wheat-fallow cropping system. J Environ Qual 27:1105–1116
Knapp AK, Smith MD (2001) Variation among biomes in temporal dynamics of aboveground primary production. Science 291:481–484
Knapp AK, Fay PA, Blair JM, Collins SL, Smith MD, Carlisle JD, Harper CW, Danner BT, Lett MS, McCarron JK (2002) Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science 298:2202–2205
Kozlowski TT, Pallardy SG (1997) Physiology of woody plants. Academic Press, San Diego, Calif.
Kropfl AI, Cecchi GA, Villasuso NM, Distel RA (2002) The influence of Larrea divaricata on soil moisture and on water status and growth of Stipa tenuis in southern Argentina. J Arid Environ 52:29–35
Lambers H, Chapin FS III, Pons TL (1998) Plant physiological ecology. Springer, Berlin Heidelberg New York, p 540
Law BE, Falge E, Gu L et al. (2002) Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agric For Meteorol 113:97–120
Le Houerou HN, Bingham RL, Skerbek W (1998) Relationship between the variability of primary production and the variability of annual precipitation in world arid lands. J Arid Environ 15:1–18
Leffler AJ, Ryel RJ, Hipps L, Ivans S, Caldwell MM (2002) Carbon acquisition and water use in a northern Utah Juniperus osteosperma (Utah juniper) population. Tree Physiol 22:1221–1230
Loik ME, Breshears DD, Lauenroth WK, Belnap J (2004) A multi-scale perspective of water pulses in dryland ecosystems: climatology and ecohydrology of the western USA. Oecologia. DOI 10.1007/s00442-004-1570-y
Mansfield TA, Hetherington AM, Atkinson CJ (1990) Some current aspects of stomatal physiology. Annu Rev Plant Physiol Plant Mol Biol 41:55–75
McAuliffe JR (1994) Landscape evolution, soil formation and ecological patterns and processes in Sonoran Desert bajadas. Ecol Monogr 64:111–148
McAuliffe JR (1999) The Sonoran Desert: landscape complexity and ecological diversity. In: Robichaux R (ed) Ecology of Sonoran Desert plants and communities. University of Arizona Press, Tucson, Ariz., pp 87–104
McAuliffe JR (2003) The atmosphere–biosphere interface: the importance of soils in arid and semi-arid environments. In: Weltzin JF, McPherson GR, (eds) Changing precipitation regimes in terrestrial ecosystems: a North American perspective. University of Arizona Press, Tucson, Ariz.
Mielnick P, Dugas WA, Mitchell, K, Havstad K (in press) Long-term measurement of CO2 flux and evapotranspiration in a Chihuahuan Desert grassland. J Arid Environ
Monger HC, Gallegos RA (2000) Biotic and abiotic processes and rates of pedogenic carbonate accumulation in the southwestern United States—relationship to atmospheric CO2 sequestration. In: Lal R, Kimbel JM, Eswaran H, Stewart BA (eds) Global climate change and pedogenic carbonates. CRC, Boca Raton, Fla., pp 273–289
Monson RK, Turnipseed AA, Sparks JP, Harley PC, Scott-Denton LE, Sparks K, Huxman TE (2002) Carbon sequestration in a high-elevation subalpine forest. Global Change Biol 8:459–478
Mooney HA (1972) The carbon balance of plants. Annu Rev Ecol Syst 3:315–346
Mooney HA, Billings WD (1961) Comparative physiological ecology of arctic and alpine populations of Oxyria digyna. Ecol Monogr 31:1–29
Nobel PS (1976) Water relations and photosynthesis of a desert CAM plant, Agave deserti. Plant Physiol 58:576–582
Nobel PS (1988) Environmental biology of agaves and cacti. Cambridge University Press, Cambridge, p 270
Nobel PS (1994) Root-soil responses to water pulses in dry environments. In: Caldwell MM, Pearcy RW (eds) Exploitation of environmental heterogeneity by plants. Academic Press, New York, pp 285–304
Nobel PS, Sanderson J (1984) Rectifier-like activities of roots of two desert succulents. J Exp Bot 35:727–737
North GB, Nobel PS (1991) Changes in hydraulic conductivity and anatomy caused by drying and rewetting roots of Agave deserti (Agavaceae). Am J Bot 78:906–915
Noy-Meir E (1973) Desert ecosystems: environment and producers. Annu Rev Ecol Syst 4:23–51
Ogle K, Reynolds JF (2004) Plant responses to precipitation in desert ecosystems: integrating functional types, pulses, thresholds, and delays. Oecologia. DOI 10.1007/s00442-004-1507-5
Osmond CB, Austin MP, Berry JA, Billings WD, Boyer JS, Dacey JWH, Nobel PS, Smith SD, Winner WE (1987) Stress physiology and the distribution of plants. Bioscience 37:38–48
Parker KC (1995) Effects of complex geomorphic history on soil and vegetation patterns on arid alluvial fans. J Arid Environ 30:19–39
Passioura JB (1988) Water transport in and to roots. Annu Rev Plant Physiol Plant Mol Biol 39:245–265
Pearcy RW (1990) Sunflecks and photosynthesis in plant canopies. Annu Rev Plant Physiol Plant Mol Biol 41:421–453
Potts DL, Williams DG (2004) Response of tree ring holocellulose δ13C to moisture availability in Populus fremontii at perennial and intermittent stream reaches. West N Am Nat 64:27–37
Reynolds JF, Kemp PR, Ogle K, Fernandez RJ (2004) Modifying the “pulse-reserve” paradigm for deserts of North America: precipitation pulses, soil water and plant responses. Oecologia. DOI 10.1007/s00442-004-1524-4
Richards JH, Caldwell MM (1987) Hydraulic lift—substantial nocturnal water transport between soil layers by Artemisia tridentata roots. Oecologia 73:486–489
Ryel RJ, Caldwell MM, Yoder CK, Or D, Leffler AJ (2002) Hydraulic redistribution in a stand of Artemisia tridentata: evaluation of benefits to transpiration assessed with a simulation model. Oecologia 130:173–184
Sala OE, Lauenroth WK (1982) Small rainfall events: an ecological role in semiarid regions. Oecologia 53:301–304
Sala OE, Lauenroth WK, Parton WJ (1982) Plant recovery following prolonged drought in a shortgrass steppe. Agric Meteorol 27:49–58
Schlesinger WH (1985) The formation of caliche in soils of the Mojave Desert, California. Geochim Comochim Acta 49:57–66
Schlesinger WH, Reynolds JF, Cunningham GL, Hueneke LF, Jarrell WM, Virginia RA, Whitford WG (1990) Biological feedbacks in global desertification. Science 247:1043–1048
Schulze ED, Caldwell MM, Canadell J, Mooney HA, Jackson RB, Parson D, Scholes R, Sala OE, Trimborn P (1998) Downward flux of water through roots (i.e., inverse hydraulic lift) in dry Kalahari sands. Oecologia 115:460–462
Schwinning S, Ehleringer JR (2001) Water use trade-offs and optimal adaptations to pulse-driven arid ecosystems. J Ecol 89:464–480
Schwinning S, Sala OE (2004) Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia. DOI 10.1007/s00442-004-1520-8
Schwinning S, Davis K, Richardson L, Ehleringer JR (2002) Deuterium enriched irrigation indicates different forms of rain use in shrub/grass species of the Colorado Plateau. Oecologia 130:345–355
Scott RL, Edwards EA, Shuttleworth WJ, Huxman TE, Watts C, Goodrich DC (2004) Interannual and seasonal variation in fluxes of water and CO2 from a riparian woodland ecosystem. Agric For Meteorol 122:65–84
Searcy JK (1959) Flow-duration curves. U.S. Geological Survey water supply paper 1542-A. U.S. Geological Survey, Washington, D.C.
Smakhtin VU (2001) Low flow hydrology: a review. J Hydrol 240:147–186
Smith WK, Knapp AK (1990) Ecophysiology of high elevation forests. In: Osmond CB, Pitelka LF, Hidy GM (eds) Plant biology of the basin and range. Springer, Berlin Heidelberg New York, pp 87–142
Smith SD, Nobel PS (1986) Deserts. In: Baker NR, Long SP (eds) Photosynthesis in contrasting environments. Topics in photosynthesis, vol 7. Elsevier, Amsterdam, pp 13–62
Smith SD, Herr CA, Leary KL, Piorkowski JM (1995) Soil–plant water relations in a Mojave Desert mixed shrub community: a comparison of three geomorphic surfaces. J Arid Environ 29:339–351
Smith SD, Monson RK, Anderson JE (1997) Physiological ecology of North American desert plants. Springer, Berlin Heidelberg New York
Smith DM, Jackson NA, Roberts JM, Ong CK (1999) Reverse flow of sap in tree roots and downward siphoning of water by Grevillea robusta. Funct Ecol 13:256–264
Snyder KA, Donovan LA, James JJ, Tiller RL, Richards JH (2004) Extensive summer water pulses do not necessarily lead to canopy growth of Great Basin and northern Mojave Desert shrubs. Oecologia. DOI 10.1007/s00442-003-1403-4
Sperry JS, Adler FR, Campbell GS, Comstock JP (1998) Limitation of plant water use by rhizosphere and xylem conductance: results from a model. Plant Cell Environ 21:347–359
Szarek SR, Ting IP (1975) Physiological responses to rainfall in Opuntia basilaris (Cactaceae). Am J Bot 62:602–609
Tang J, Baldocchi DD, Qi Y, Xu L (2003) Assessing soil CO2 efflux using continuous measurements of CO2 profiles in soils with small solid-state sensors. Agric For Meteorol 118:207–220
Tromble J (1988) Water interception by two arid land shrubs. J Arid Environ 15:65–70
Valentini R, Matteucci G, Dolman AJ et al (2000) Respiration as the main determinant of carbon balance in European forests. Nature 404:861–865
Vinton MA, Burke IC (1995) Interactions between individual plant species and soil nutrient status in shortgrass steppe. Ecology 76:1116–1133
Vogel RM, Fennessey NM (1995) Flow duration curves II: a review of applications in water resources planning. Water Resour Bull 31:1029–1039
Wainwright J, Parsons AJ, Schlesinger WH, Abrahams AD (2002) Hydrology–vegetation interactions in areas of discontinuous flow on a semi-arid bajada, southern New Mexico. J Arid Environ 51:319–338
Webb W, Szareck S, Lauenroth W, Kinerson R, Smith M (1978) Primary productivity and water-use in native forest, grassland, and desert ecosystems. Ecology 59:1239–1247
Weltzin JF, Tissue DT (2003) Resource pulses in arid environments—patterns of rain, patterns of life. New Phytol 157:171–173
Weltzin JF, Loik ME, Schwinning S, Williams DG, Fay P, Haddad B, Harte J, Huxman TE, Knapp AK, Lin G, Pockman WT, Shaw MR, Small E, Smith MD, Smith SD, Tissue DT, Zak JC (2003) Assessing the response of terrestrial ecosystems to potential changes in precipitation. BioScience 53:941–952
Whitford WG (2002) Ecology of desert systems. Academic Press, San Diego, Calif.
Whitford WG, Anderson J, Rice PM (1997) Stemflow contribution to the “fertile island” effect in creosotebush; Larrea tridentata. J Arid Environ 35:451–457
Williams DG, Ehleringer JR (2000) Intra- and interspecific variation for summer precipitation use in pinyon-juniper woodlands. Ecol Monogr 70:517–537
Xu L, Baldocchi DD (2004) Seasonal variation in carbon dioxide exchange over a Mediterranean annual grassland in California. Agric For Meteorol 123:79–96
Yan S, Wan C, Sosebee RE, Wester DB, Fish EB, Zartman RE (2000) Responses of photosynthesis and water relations to rainfall in the desert shrub creosote bush (Larrea tridentata) as influenced by municipal biosolids. J Arid Environ 46:397–412
Zhang J, Davies WJ (1990) Changes in the concentration of ABA in xylem sap as a function of changing soil water status can account for changes in leaf conductance and growth. Plant Cell Environ 13:277–286
Acknowledgements
The authors would like to acknowledge the support of the United States National Science Foundation grant NSF-DEB no. 0222313 (supporting the workshop from which these ideas developed), NSF-DEB-0129326 (D. R. S.), the Biological and Environmental Research (B. E. R.) Program, United States Department of Energy, through the Southcentral Regional Center of NIGEC (W. T. P.), the International Arid Lands Consortium (T. H. E.) and the University of Arizona. This material is based upon work supported in part by Sustainability of Semiarid Hydrology and Riparian Areas (SAHRA) under the STC Program of the National Science Foundation, agreement no. EAR-9876800. D. L. Potts was supported by CATTS, a University of Arizona/NSF GK-12 program. We thank all the participants of the workshop Resource Pulse Utilization in Arid and Semiarid Ecosystems for stimulating discussion that prompted the consideration of the role of precipitation pulses on the C balance of deserts and desert organisms, and J. R. Ehleringer, M. E. Loik, and O. E. Sala for organizing the meeting.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
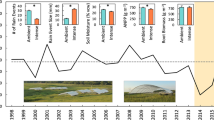
We compiled flux duration curves [analogous to the stream flow duration curves (Searcy 1959)], to illustrate the differences in ecosystem CO2 exchange characteristics for a pulsed ecosystem [a desert grassland (Jornada Experimental Range; Mielnick et al., in press)] and an ecosystem that experiences a relatively steady-state decline in soil water availability in time [a coniferous forest (Niwot Ridge AMERIFLUX site; Monson et al. 2002)]. We used 30- and 20-min averaged (Niwot Ridge and Jornada, respectively) peak growing season (June–August) NEE values observed over 4 years (1999–2002 and 1997–2000, Niwot Ridge and Jornada, respectively). Briefly, NEE data for the period of interest at each site were assigned a rank (r) in order of descending magnitude, positive to negative. A probability of exceedance (F) was calculated for each ranked NEE value (r) according to the formula:
where n is the number of ranked NEE values for the period of interest. Like flow duration analysis in hydrology (Searcy 1959; Vogel and Fennessy 1995; Potts and Williams 2004), flux duration analysis provides a convenient and repeatable standard for comparing patterns of ecosystem exchange between sites and between years at the same site. By ranking and assigning a frequency to ecosystem exchange values, flux duration analysis incorporates episodic high activity periods, such as those associated with precipitation pulses, and sustained low level fluxes during interpulse periods into a single calculation. As additional ecosystem scale flux data sets become available, it may be possible to broadly classify ecosystem flux duration curves as “pulsed-dominated” and “steady-state” similarly to the way hydrograph-derived flow duration curves can be described and classified by the physical, biotic and anthropogenic factors controlling stream flow (e.g., Vogel and Fennessey 1995; Smakhtin 2001).
Rights and permissions
About this article
Cite this article
Huxman, T.E., Snyder, K.A., Tissue, D. et al. Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 141, 254–268 (2004). https://doi.org/10.1007/s00442-004-1682-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1682-4