Abstract
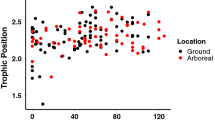
For diverse communities of omnivorous insects such as ants, the extent of direct consumption of plant-derived resources vs. predation is largely unknown. However, determination of the extent of "herbivory" among ants may be crucial to understand the hyper-dominance of ants in tropical tree crowns, where prey organisms tend to occur scarcely and unpredictably. We therefore examined N and C stable isotope ratios (δ15N and δ13C) in 50 ant species and associated insects and plants from a tropical rainforest in North Queensland, Australia. Variation between ant species was pronounced (range of species means: 7.1‰ in δ15N and 6.8‰ in δ13C). Isotope signatures of the entire ant community overlapped with those of several herbivorous as well as predacious arthropods. Variability in δ15N between ants was not correlated with plant δ15N from which they were collected. Ant species spread out in a continuum between largely herbivorous and purely predacious taxa, with a high degree of omnivory. Ant species' δ15N were consistent with the trophic level predicted by natural feeding observations, but not their δ13C. Low δ15N levels were recorded for ant species that commonly forage for nectar on understorey or canopy plants, intermediate levels for species with large colonies that were highly abundant on nectar and honeydew sources and were predacious, and the highest levels for predominantly predatory ground-foraging species. Colonies of the dominant weaver-ants ( Oecophylla smaragdina) had significantly lower δ15N in mature forests (where preferred honeydew and nectar sources are abundant) than in open secondary vegetation. N concentration of ant dry mass showed only very limited variability across species and no correlation with trophic levels. This study demonstrates that stable isotopes provide a powerful tool for quantitative analyses of trophic niche partitioning and plasticity in complex and diverse tropical omnivore communities.


Similar content being viewed by others
References
Andersen AN (1995) A classification of Australian ant communities, based on functional groups which parallel plant life-forms in relation to stress and disturbance. J Biogeogr 22:15–29
Bauer GA, Gebauer G, Harrison AF, Högberg P, Högbom L, Schinkel H, Taylor AFS, Novak M, Buzek F, Harkness D, Persson T, Schulze E-D (2000) Biotic and abiotic controls over ecosystem cycling of stable natural nitrogen, carbon and sulphur isotopes. In: Schulze E-D (ed) Carbon and nitrogen cycling in European forest ecosystems, vol 142. Springer, Berlin Heidelberg New York, pp 189–214
Beattie AJ (1985) The evolutionary ecology of ant-plant mutualisms. Cambridge University Press, New York
Blüthgen N (2003) How availability and quality of nectar and honeydew shape an Australian rainforest ant community. PhD thesis. University of Bayreuth, Bayreuth
Blüthgen N, Fiedler K (2002) Interactions between weaver ants ( Oecophylla smaragdina), homopterans, trees and lianas in an Australian rainforest canopy. J Anim Ecol 71:793–801
Blüthgen N, Verhaagh M, Goitía W, Jaffé K, Morawetz W, Barthlott W (2000) How plants shape the ant community in the Amazonian rainforest canopy: the key role of extrafloral nectaries and homopteran honeydew. Oecologia 125:229–240
Bonal D, Sabatier D, Montpied P, Tremeaux D, Guehl JM (2000) Interspecific variability of δ13C among trees in rainforests of French Guiana: functional groups and canopy integration. Oecologia 124:454–468
Boutton TW, Arshard MA, Tieszen LL (1983) Stable isotope analysis of termite food habits in East African grasslands. Oecologia 59:1–6
Briese DT, Macauley BJ (1981) Food collection within an ant community in semi-arid Australia, with special reference to seed harvesters. Aust J Ecol 6:1–19
Cabana G, Rasmussen JB (1994) Modelling food chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature 372:255–257
Carver M, Gross GF, Woodward TE (1994) Hemiptera. In: Naumann ID (ed) Systematic and applied entomology. Melbourne University Press, Melbourne, pp 316–329
Davidson DW (1997) The role of resource imbalances in the evolutionary ecology of tropical arboreal ants. Biol J Linn Soc 61:153–181
Davidson DW, Patrell-Kim L (1996) Tropical arboreal ants: why so abundant? In: Gibson AC (ed) Neotropical biodiversity and conservation. Mildred E. Mathias Botanical Garden, University of California, Los Angeles, Calif., pp 127–140
Davidson DW, Cook SC, Snelling RR, Chua TH (2003) Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300:969–972
De Niro MJ, Epstein S (1978) Influence of the diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
De Niro MJ, Epstein S (1981) Influence of the diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Dejean A, McKey D, Gibernau M, Belin M (2000) The arboreal ant mosaic in a Cameroonian rainforest (Hymenoptera: Formicidae). Sociobiology 35:403–423
Fischer RC, Richter A, Wanek W, Mayer V (2002) Plants feed ants: food bodies of myrmecophytic Piper and their significance for the interaction with Pheidole bicornis ants. Oecologia 133:186–192
Fisher BL, Sternberg LdSL, Price D (1990) Variation in the use of orchid extrafloral nectar by ants. Oecologia 83:263–266
Floren A, Biun A, Linsenmair KE (2002) Arboreal ants as key predators in tropical lowland rainforest trees. Oecologia 131:137–144
Gannes LZ, O'Brien DM, Martinez del Rio C (1997) Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology 78:1271–1276
Gearing JN, Gearing PJ, Rudnick DT, Requejo AG, Hutchins MJ (1984) Isotopic variability of organic carbon in a phytoplankton-based estuary. Geochim Cosmochim Acta 48:1089–1098
Gebauer G, Schulze E-D (1991) Carbon and nitrogen isotope ratios in different compartments of a healthy and a declining Picea abies forest in the Fichtelgebirge, NE Bavaria. Oecologia 87:198–207
Gleixner G, Danier HJ, Werner RA, Schmidt HL (1993) Correlations between the 13-C content of primary and secondary plant products in different cell compartments and that in decomposing basidiomycetes. Plant Physiol 102:1287–1290
Guehl JM, Domenach AM, Bereau M, Barigah TS, Casabianca H, Ferhi A, Garbaye J (1998) Functional diversity in an Amazonian rainforest of French Guyana: a dual isotope approach (δ15Nand δ13C). Oecologia 116:316–330
Haack KD, Vinson SB, Olson JK (1995) Food distribution and storage in colonies of Monomorium pharaonis (L.) (Hymenoptera, Formicidae). J Entomol Sci 30:70–81
Hendrix PF, Lachnicht SL, Callaham MA, Zou X (1999) Stable isotopic studies of earthworm feeding ecology in tropical ecosystems of Puerto Rico. Rapid Comm Mass Spec 13:1295–1299
Hobson KA (1999) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120:314–326
Högberg P (1997) 15N natural abundance in soil-plant systems. New Phytol 137:179–203
Hölldobler B (1983) Territorial behavior in the green tree ant ( Oecophylla smaragdina). Biotropica 15:241–250
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge, Mass.
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27
May RM (1973) Stability and complexity in model ecosystems. Princeton University Press, Princeton, N.J.
McCann K, Hastings A, Huxel GR (1998) Weak trophic interactions and the balance of nature. Nature 395:794–798
Mingawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 50:2143–2146
Nagy L, Proctor J (2000) Leaf δ13C signatures in heath and lowland evergreen rain forest species from Borneo. J Trop Ecol 16:757–761
Ostrom PH, Colunga-Garcia M, Gage SH (1997) Establishing pathways of energy flow for insect predators using stable isotope ratios: field and laboratory evidence. Oecologia 109:108–113
Peng RK, Christian K, Gibb K (1999) The effect of colony isolation of the predacious ant, Oecophylla smaragdina (F.) (Hymenoptera: Formicidae), on protection of cashew plantations from insect pests. Int J Pest Manage 45:189–194
Pimm SL, Lawton JH, Cohen JE (1991) Food web patterns and their consequences. Nature 350:669–674
Ponsard S, Arditi R (2000) What can stable isotopes (δ15N and δ13C) tell about the food web of soil macro-invertebrates? Ecology 81:852–864
Ponsard S, Averbuch P (1999) Should growing and adult animals fed on the same diet show different δ15N values? Rapid Commun Mass Spectrom 13:1305–1310
Post DM (2002a) The long and the short of food-chain length. Trends Ecol Evol 17:269–277
Post DM (2002b) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Reagan DP, Waide RB (eds) (1996) The food web of a tropical rain forest. University of Chicago Press, Chicago, Ill.
Rico-Gray V, Sternberg LDSL (1991) Carbon isotopic evidence for seasonal change in feeding habits of Camponotus planatus Roger (Formicidae) in Yucatan, Mexico. Biotropica 23:93–95
Sagers CL, Ginger SM, Evans RD (2000) Carbon and nitrogen isotopes trace nutrient exchange in an ant-plant mutualism. Oecologia 123:582–586
Scheu S, Falca M (2000) The soil food web of two beech forests ( Fagus sylvatica) of contrasting humus type: stable isotope analysis of a macro- and a mesofauna-dominated community. Oecologia 123:285–296
Schmidt S, Stewart GR (2003) δ15N values of tropical savanna and monsoon forest species reflect root specializations and soil nitrogen status. Oecologia 134:569–577
Shattuck SO (1999) Australian ants: their biology and identification. CSIRO, Collingwood, Victoria
Stork NE (1991) The composition of the arthropod fauna of Bornean lowland rain forest trees. J Trop Ecol 7:161–180
Stradling DJ (1978) Food and feeding habits of ants. In: Brian MV (ed) Production ecology of ants and termites, vol 13. Cambridge University Press, Cambridge, pp 81–106
Tayasu I (1998) Use of carbon and nitrogen isotope ratios in termite research. Ecol Res 13:377–387
Tayasu I, Abe T, Eggleton P, Bignell DE (1997) Nitrogen and carbon isotope ratios in termites: an indicator of trophic habit along the gradient from wood-feeding to soil-feeding. Ecol Entomol 22:343–351
Tobin JE (1991) A neotropical rainforest canopy, ant community: some ecological considerations. In: Huxley CR, Cutler DF (eds) Ant-plant interactions. Oxford University Press, Oxford, pp 536–538
Tracey JG (1982) The vegetation of the humid tropical region of North Queensland. CSIRO, Melbourne
Vinson SB (1968) The distribution of an oil, carbohydrate, and protein food source to members of the imported fire ant colony. J Econ Entomol 61:712–714
Way MJ (1953) The relationship between certain ant species with particular reference to biological control of the coreid, Theraptus sp. Bull Entomol Res 44:669–691
Winter K, Wallace BJ, Stocker GC, Roksandic Z (1983) Crassulacean acid metabolism in Australian vascular epiphytes and some related species. Oecologia 57:129–141
Acknowledgements
We thank Marga Wartinger for technical assistance in the isotope analyses. Taxonomic identification of ants and homopterans was kindly supported by Mary Carver (aphids), Max Day (membracids), John Donaldson (coccoids), Brian Heterick ( Monomorium), Rudy Kohout ( Polyrhachis), Hanna Reichel ( Rhytidoponera), Steve Shattuck (several ant genera) and Mike Webb (cicadellids). Identification of plants was supported on site or in the herbarium by Bruce Gray, Bernie Hyland, Bob Jago, Rigel Jensen and Andrew Small. Diane Davidson, Carlos Martínez del Rio and two anonymous referees provided helpful comments that improved the manuscript. We thank Nigel Stork for his intensive support in Australia and Peter Franks for preliminary isotope analyses in order to evaluate the method. The Australian Canopy Crane Company and the Environmental Research Station in Cape Tribulation provided valuable logistic support. Financial support was provided by the Deutsche Forschungsgemeinschaft (Fi 547/9–1) and by a doctoral fellowship of the Studienstiftung des deutschen Volkes to N. B.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
For ant, plant and homopteran species and their codes used in Fig. 1 see Table 1
Rights and permissions
About this article
Cite this article
Blüthgen, N., Gebauer, G. & Fiedler, K. Disentangling a rainforest food web using stable isotopes: dietary diversity in a species-rich ant community. Oecologia 137, 426–435 (2003). https://doi.org/10.1007/s00442-003-1347-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1347-8