Abstract
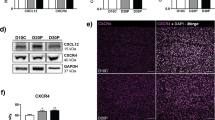
Chemokines play a significant role in pregnancy, especially during embryonic attachment and placental development. During early pregnancy, immune cells are recruited extensively to the endometrium in several species including pigs. However, this recruitment is solely mediated by the presence of the conceptus in pigs making it a unique feature compared with other species (humans, primates and mice). To understand the biological significance of chemokine expression and immune cell recruitment in the context of fetal loss, we investigate a well-characterized porcine fetal loss model during the window of early pregnancy at gestational day (gd) 20 and mid-pregnancy (gd50). These periods coincide with 25–40 % of conceptus loss. Using targeted quantitative polymerase chain reaction and Western blot approaches, we screened a specific set of chemokines. Comparisons were made with endometrial lymphocytes (ENDO LY), endometrium and chorioallantoic membranes (CAM) associated with spontaneously arresting and healthy conceptus attachment sites (CAS). mRNA expression studies revealed an increased expression of CXCR3 and CCR5 in ENDO LY and of CXCL10, CXCR3, CCL5 and CCR5 in the endometrium associated with arresting CAS at gd20. DARC was decreased in the endometrium at gd50. CCL1 was increased in CAM associated with arresting CAS at gd50. Some of these differences were also noted at the protein level (CXCL10, CXCR3, CCL5 and CCR5) in the endometrium and CAM. CD45+ immunohistochemistry demonstrated a significantly higher localization in ENDO LY in the endometrium associated with healthy versus arresting counterparts. Most of these differences were observed in early pregnancy and might contribute towards a shift in immune cell functions.






Similar content being viewed by others
Abbreviations
- CAM:
-
Chorioallantoic membrane
- CAS:
-
Conceptus attachment sites
- ENDO LY:
-
Endometrial lymphocytes
- LCM:
-
Laser capture microdissection
References
Angiolillo AL, Sgadari C, Taub DD, Liao F, Farber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G (1995) Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med 182:155–162
Bazer FW, Johnson GA (2014) Pig blastocyst-uterine interactions. Differentiation 87:52–65
Bidarimath M, Khalaj K, Wessels JM, Tayade C (2014) MicroRNAs, immune cells and pregnancy. Cell Mol Immunol 11:538–547
Bidarimath M, Edwards AK, Tayade C (2015a) Laser capture microdissection for gene expression analysis. Methods Mol Biol 1219:115–137
Bidarimath M, Edwards AK, Wessels JM, Khalaj K, Kridli RT, Tayade C (2015b) Distinct microRNA expression in endometrial lymphocytes, endometrium, and trophoblast during spontaneous porcine fetal loss. J Reprod Immunol 107:64–79
Bischof RJ, Brandon MR, Lee C-S (1995) Cellular immune responses in the pig uterus during pregnancy. J Reprod Immunol 29:161–178
Charo IF, Ransohoff RM (2006) The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354:610–621
Croy BA, Waterfield A, Wood W, King GJ (1988) Normal murine and porcine embryos recruit NK cells to the uterus. Cell Immunol 115:471–480
Croy BA, Wessels J, Linton N, Tayade C (2009) Comparison of immune cell recruitment and function in endometrium during development of epitheliochorial (pig) and hemochorial (mouse and human) placentas. Placenta 30 (Suppl A):S26–S31
Dantzer V, Leiser R (1994) Initial vascularisation in the pig placenta. I. Demonstration of nonglandular areas by histology and corrosion casts. Anat Rec 238:177–190
Dimova T, Mihaylova A, Spassova P, Georgieva R (2007) Establishment of the porcine epitheliochorial placenta is associated with endometrial T-cell recruitment. Am J Reprod Immunol 57:250–261
Dimova T, Mihaylova A, Spassova P, Georgieva R (2008) Superficial implantation in pigs is associated with decreased numbers and redistribution of endometrial NK-cell populations. Am J Reprod Immunol 59:359–369
Du MR, Wang SC, Li DJ (2014) The integrative roles of chemokines at the maternal-fetal interface in early pregnancy. Cell Mol Immunol 11:438–448
Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD (2002) IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol 168:3195–3204
Engelhardt H, Croy BA, King GJ (2002) Conceptus influences the distribution of uterine leukocytes during early porcine pregnancy. Biol Reprod 66:1875–1880
Erlebacher A (2010) Immune surveillance of the maternal/fetal interface: controversies and implications. Trends Endocrinol Metab 21:428–434
Erlebacher A (2013) Immunology of the maternal-fetal interface. Annu Rev Immunol 31:387–411
Geisert RD, Lucy MC, Whyte JJ, Ross JW, Mathew DJ (2014) Cytokines from the pig conceptus: roles in conceptus development in pigs. J Anim Sci Biotechnol 5:51
Gombert M, Dieu-Nosjean M-C, Winterberg F, Bünemann E, Kubitza RC, Da Cunha L, Haahtela A, Lehtimäki S, Müller A, Rieker J, Meller S, Pivarcsi A, Koreck A, Fridman W-H, Zentgraf H-W, Pavenstädt H, Amara A, Caux C, Kemeny L, Alenius H, Lauerma A, Ruzicka T, Zlotnik A, Homey B (2005) CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. J Immunol 174:5082–5091
Gonzalo JA, Qiu Y, Lora JM, Al-Garawi A, Villeval JL, Boyce JA, Martinez-A C, Marquez G, Goya I, Hamid Q, Fraser CC, Picarella D, Cote-Sierra J, Hodge MR, Gutierrez-Ramos JC, Kolbeck R, Coyle AJ (2007) Coordinated involvement of mast cells and T cells in allergic mucosal inflammation: critical role of the CC chemokine ligand 1:CCR8 axis. J Immunol 179:1740–1750
Griffith JW, Sokol CL, Luster AD (2014) Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 32:659–702
Hannan NJ, Salamonsen LA (2007) Role of chemokines in the endometrium and in embryo implantation. Curr Opin Obstet Gynecol 19:266–272
Khalaj K, Wessels JM, Kridli RT, Bidarimath M, LaMarre J, Tayade C (2015) mRNA destabilizing factors: tristetraprolin expression at the porcine maternal-fetal interface. Am J Reprod Immunol 73:402–416
Luster AD, Unkeless JC, Ravetch JV (1985) Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 315:672–6
Mantovani A (1999) The chemokine system: redundancy for robust outputs. Immunol Today 20:254–257
Mehrad B, Keane MP, Strieter RM (2007) Chemokines as mediators of angiogenesis. Thromb Haemost 97:755–62
Moffett A, Loke C (2006) Immunology of placentation in eutherian mammals. Nat Rev 6:584–594
Moffett-King A (2002) Natural killer cells and pregnancy. Nat Rev 2:656–663
Owen JL, Mohamadzadeh M (2013) Macrophages and chemokines as mediators of angiogenesis. Front Physiol 4:159
Petit I, Jin D, Rafii S (2007) The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol 28:299–307
Ross JW, Ashworth MD, Stein DR, Couture OP, Tuggle CK, Geisert RD (2009) Identification of differential gene expression during porcine conceptus rapid trophoblastic elongation and attachment to uterine luminal epithelium. Physiol Genomics 36:140–148
Stroband HW, Van der Lende T (1990) Embryonic and uterine development during early pregnancy in pigs. J Reprod Fertil 40:261–277
Tayade C, Black GP, Fang Y, Croy BA (2006) Differential gene expression in endometrium, endometrial lymphocytes, and trophoblasts during successful and abortive embryo implantation. J Immunol 176:148–156
Tayade C, Fang Y, Hilchie D, Croy BA (2007) Lymphocyte contributions to altered endometrial angiogenesis during early and midgestation fetal loss. J Leukoc Biol 82:877–886
Tayade C, Edwards AK, Bidarimath M (2014) Laser capture microdissection. In: Croy BA, Yamada AT, DeMayo FJ, Adamson SL (eds) The guide to investigation of mouse pregnancy. Academic Press (Elsevier), London, pp 567–575
Vega CJ (2008) Laser microdissection sample preparation for RNA analyses. Methods Mol Biol 414:241–252
Wessels JM, Linton NF, Croy BA, Tayade C (2007) A review of molecular contrasts between arresting and viable porcine attachment sites. Am J Reprod Immunol 58:470–480
Wessels JM, Linton NF, Heuvel MJ van den, Cnossen SA, Edwards AK, Croy BA, Tayade C (2011) Expression of chemokine decoy receptors and their ligands at the porcine maternal-fetal interface. Immunol Cell Biol 89:304–313
Wessels JM, Khalaj K, Kridli RT, Edwards AK, Bidarimath M, Tayade C (2014) Are pharmacological interventions between conception and birth effective in improving reproductive outcomes in North American swine? Reprod Domest Anim 49:536–542
Winther H, Ahmed A, Dantzer V (1999) Immunohistochemical localization of vascular endothelial growth factor (VEGF) and its two specific receptors, Flt-1 and KDR, in the porcine placenta and non-pregnant uterus. Placenta 20:35–43
Wu X, Jin L-P, Yuan M-M, Zhu Y, Wang M-Y, Li D-J (2005) Human first-trimester trophoblast cells recruit CD56brightCD16- NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol 175:61–68
Yates-Binder CC, Rodgers M, Jaynes J, Wells A, Bodnar RJ, Turner T (2012) An IP-10 (CXCL10)-derived peptide inhibits angiogenesis. PLoS One 7:e40812
Acknowledgments
The authors thank Dr. Stephen C. Pang for access to the real-time PCR facilities at the Department of Biomedical and Molecular Sciences, Queen’s University. The support from technical staff at the Arkell Swine Research Station and the Meat Wing of the Animal and Poultry Science at the University of Guelph is gratefully acknowledged. We also thank Allison Felker at Queen’s University for technical assistance with the immunofluorescence technique.
Author information
Authors and Affiliations
Corresponding author
Additional information
Generous funding support from the NSERC-CRD, Ontario Pork, OMAFRA and Bioniche Life Sciences (C.T.) and via the Queen’s Graduate Award, Queen’s University (M.B.) is greatly appreciated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
Protein expression of chemokines and their receptors in chorioallantoic membrane at gd20. Chemokines (CCL5, CCR5, CXCL10, CXCR3 and CCL1) that were significantly different between healthy and arresting conceptus attachment sites (CAS) at gd20 were quantified. Relative quantification was performed by using ACTB as the control protein. Each healthy and arresting group consisted in n = 12 per group and n = 6 sample type (n = 6 HCAM, n = 6 ACAM). Densitometry analysis was performed to give a ratio of chemokine proteins to ACTB by using Image J. No significant differences (P < 0.05) were evident in the expression of selected chemokines and their receptors at the protein level in the ACAM compared with HCAM at gd20 (IDV individual densitometry values). Densitometric values are presented as means ± SEM. *Statistical significance at P < 0.05. (GIF 114 kb)
Supplemental Figure 2
Protein expression of chemokines and their receptors in chorioallantoic membrane at gd50. Chemokines (CCL5, CCR5, CXCL10, CXCR3 and CCL1) that were significantly different between healthy and arresting conceptus attachment sites (CAS) were quantified in chorioallantoic membranes collected at gd50. Relative quantification was performed by using ACTB as the reference protein. Each healthy and arresting group consisted in n = 12 per group and n = 6 sample type (n = 6 HCAM, n = 6 ACAM). Densitometry analysis was performed to give a ratio of chemokine to ACTB by using Image J. Relative expression of chemokine proteins (CCL5, CCR5, CXCL10, CXCR3 and CCL1) between HCAM and ACAM did not differ significantly at gd50 (IDV individual densitometry values). Densitometric values are presented as means ± SEM. *Statistical significance at P < 0.05. (GIF 114 kb)
Supplemental Figure 3
Immunolocalization of Foxp3+ cells in the porcine endometrium at gd20. Left and right columns represent the distribution of Foxp3+ cells in the endometrium associated with healthy (HE) and arresting (AE) CAS, respectively. Nuclei are stained with 4,6-diamidino-2-phenylindole (DAPI, blue in a, b), Foxp3+ is stained with Anti-Mouse/Rat Foxp3 PE (DsRed, red in c, d) and the merged images (e, f) demonstrate their localization in the endometrium. Magnification ×400. Bar 400 μm. (GIF 131 kb)
Rights and permissions
About this article
Cite this article
Bidarimath, M., Khalaj, K., Kridli, R.T. et al. Altered expression of chemokines and their receptors at porcine maternal-fetal interface during early and mid-gestational fetal loss. Cell Tissue Res 366, 747–761 (2016). https://doi.org/10.1007/s00441-016-2470-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2470-2