Abstract
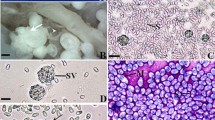
A new microsporidian infecting the connective tissue of the coelomic cavity of the blacktail comber Serranus atricauda, in the Madeira Archipelago (Portugal), is described on the basis of morphological, ultrastructural, and molecular features. The microsporidian formed large whitish xenomas adhering to the peritoneal visceral organs of the host. Each xenoma consisted of a single hypertrophic cell, in the cytoplasm of which mature spores proliferated within parasitophorous vacuoles surrounded by numerous collagen fibers. Mature spores were ellipsoidal and uninucleated, measuring an average of 6.5 ± 0.5 μm in length and 3.4 ± 0.6 μm in width. The anchoring disk of the polar filament was subterminal, laterally shifted from the anterior pole of the spore. The isofilar polar filament coiled in 18–19 turns, forming two rows that surrounded the posterior vacuole. The latter occupied about one third of the spore length. The polaroplast surrounding the apical and uncoiled portion of the polar filament displayed two distinct regions: a lamellar region and an electron-dense globule. Molecular analysis of the rRNA genes, including the internal transcribed spacer region, and phylogenetic analysis using maximum likelihood and neighbor joining demonstrated that this microsporidian parasite clustered with some Glugea species. Based on the differences found both at the morphological and molecular levels, to other members of the genus Glugea, the microsporidian infecting the blacktail comber is considered a new species, thus named Glugea serranus n. sp.




Similar content being viewed by others
References
Abdel-Baki A-AS, Al-Quraishy S, Rocha S, Dkhil MA, Casal G, Azevedo C (2015a) Ultrastructure and phylogeny of Glugea nagelia sp. n. (Microsporidia: Glugeidae), infecting the intestinal wall of the yellowfin hind, Cephalopholis hemistiktos (Actinopterygii, Serranidae), from the Red Sea. Folia Parasitol 62:007, doi: 10.14411/fp.2015.007
Abdel-Baki A-AS, Tamihi AF, Al-Qahtani HA, Al-Quraishy S, Mansour L (2015b) Glugea jazanensis sp. nov. infecting Lutjanus bohar in the Red Sea: ultrastructure and phylogeny. Dis Aquat Org 116:185–190. doi:10.3354/dao02927
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. doi:10.1016/S0022-2836(05)80360-2
Azevedo C, Abdel-Baki A-A, Rocha S, Al-Quraishy S, Casal G (2016) Ultrastructure and phylogeny of Glugea arabica n. sp. (Microsporidia), infecting the marine fish Epinephelus polyphekadion from the Red Sea. Eur J Protistol 52:11–21. doi:10.1016/j.ejop.2015.09.003
Bell AS, Aoki T, Yokoyama H (2001) Phylogenetic relationships among microsporidia based on rDNA sequence data, with particular reference to fish-infecting Microsporidium Balbiani 1884 species. J Eukaryot Microbiol 48:258–265. doi:10.1111/j.1550-7408.2001.tb00313.x
Brown AMV, Kent ML, Adamson ML (2010) Description of five new Loma (Microsporidia) species in Pacific fishes with redesignation of the type species Loma morhua Morrison & Sprague, 1981, based on morphological and molecular species-boundaries tests. J Eukaryot Microbiol 57:529–553. doi:10.1111/j.1550-7408.2010.00508.x
Canning EU, Lom J (1986) The microsporidia of vertebrates. Academic, London
Canning EU, Lom J, Nicholas JP (1982) Genus Glugea Thélohan 1891 (phylum Microspora): redescription of the type species Glugea anomala (Moniez 1887) and recognition of its sporogonic development within sporophorous vesicles (pansporoblastic membranes). Protistologica 18:193–210
Diamant A, Rothman SBS, Goren M, Galil BS, Yokes MB, Szitenberg A, Huchon D (2014) Biology of a new xenoma-forming gonadotropic microsporidium in the invasive blotchfin dragonet Callionymus filamentosus. Dis Aquat Org 109:35–54. doi:10.3354/dao02718
Gatehouse HS, Malone LA (1998) The ribosomal RNA gene region of Nosema apis (Microspora): DNA sequence for small and large subunit rRNA genes and evidence of a large tandem repeat unit size. J Invertebr Pathol 71:97–105. doi:10.1111/j.1550-7408.2011.00590.x
Jithendran KP, Vijayan KK, Kailasam M (2011) Microsporidian (Glugea sp.) infection in the greasy grouper Epinephelus tauvina (Forsskal, 1775). Indian J Fish 58:125–127
Larsson JIR (1999) Identification of microsporidia. Acta Protozool 39:161–197
Leiro J, Ortega M, Iglesias R, Estevez J, Sanmartin ML (1996) Pleistophora finisterrensis n. sp., a microsporidian parasite of blue whiting Micromesistius poutassou. Syst Parasitol 34:163–170
Lom J (2002) Catalogue of described genera and species of microsporidians parasitic in fish. Syst Parasitol 53:81–99
Lom J, Dyková I (1992) Microsporidia (phylum Microspora Sprague, 1977). In: Lom J, Dyková I (eds) Protozoan parasites of fishes. Developments in Aquaculture and Fisheries Science, Amsterdam: Elsevier, p 125–157
Lom J, Laird M (1976) Parasitic protozoa from marine and euryhaline fish of Newfoundland and New Brunswick. II. Microsporidia. Trans Amer Micros Soc 95:569–580
Lom J, Nilsen F (2003) Fish microsporidia fine structural diversity and phylogeny. Intern J Parasitol 33:107–127. doi:10.1016/S0020-7519(02)00252-7
Lom J, Noga EJ, Dyková I (1995) Occurrence of a microsporean with characteristics of Glugea anomala in ornamental fish of the family Cyprinodontidae. Dis Aquat Org 21:239–242
Marzouk MS, Anter OM, Ali MM, Kenawy AM, Mahmaud MA (2010) Studies on epizootic microsporidiosis in wild dusky grouper (Epinephelus guaza) from Mediterranean Sea at Matrouh Governorate, Egypt. Egypt J Aquat Biol Fish 14:1–12
Nagasawa K, Cruz-Lacierda ER (2004) Diseases of cultured groupers. Southeast Asian Fisheries Development Center, Aquaculture Department. Government of Japan Trust Fund, Iloilo, Philippines
Nilsen F (2000) Small subunit ribosomal DNA phylogeny of microsporidia with particular reference to genera that infect fish. J Parasitol 86:128–133. doi:10.2307/3284922
Payghan R, Nabavi L, Jamshidi K, Akbari S (2009) Microsporidian infection in lizard fish, Saurida undosquamis of Persian Gulf. Iranian J Vet Res 10:180–185
Pomport-Castillon C, Jonckheere JF, Romestand B (2000) Ribosomal DNA sequences of Glugea anomala, G. stephani, G. americanus and Spraguea lophii (Microsporidia): phylogenetic reconstruction. Dis Aquat Org 40:125–129. doi:10.3354/dao040125
Rodriguez-Tovar LE, Speare DJ, Markham RJF, Daley J (2004) Predictive modeling of post-onset xenoma growth during microsporidial gill disease (Loma salmonae) of salmonids. J Comp Pathol 131:330–333. doi:10.1016/j.jcpa.2004.04.001
Sprague V, Becnel JJ, Hazard EI (1992) Taxonomy of phylum microspora. Crit Rev 18:285–395
Stentiford GD, Feist SW, Stone DM, Bateman KS, Dunn AM (2013) Microsporidia: diverse, dynamic and emergent pathogens in aquatic systems. Trends in Parasitol 29:567–578. doi:10.1016/j.pt.2013.08.005
Su Y, Feng J, Sun X, Jiang J, Guo Z, Ye L, Xu L (2014) A new species of Glugea Thélohan, 1891 in the red sea bream Pagrus major (Temminck & Schlegel) (Teleostei: Sparidae) from China. Syst Parasitol 89:175–183. doi:10.1007/s11230-014-9519-y
Tamura K, Dudley J, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Thompson JD, Higgins DG, Gilson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acid Res 22:4673–4680. doi:10.1093/nar/22.22.4673
Vagelli A, Paramá A, Sanmartín ML, Leiro J (2005) Glugea vincentiae n. sp. (Microsporidia: Glugeidae) infecting the Australian marine fish Vincentia conspersa (Teleostei: Apogonidae). J Parasitol 91:152–157. doi:10.1645/GE-388R
Vávra J, Lukeš J (2013) Microsporidia and “the art of living together”. Adv Parasitol 82:254–319. doi:10.1016/B978-0-12-407706-5.00004-6
Voronin VN, Iukhimenko SS (2010) A new microsporidian species Glugea mesocotti sp. n. (Microsporidia: Glugeidae) from Mesocottus haitej (Scorpaeniformes: Cottidae)]. Parazitologiia 44:351–355
Vossbrinck CR, Debrunner-Vossbrinck BA (2005) Molecular phylogeny of the microsporidia: ecological, ultrastructural and taxonomic considerations. Folia Parasitol 52:131–142, 10.14411/fp.2005.017
Weiss L, Vossbrinck C (1999) Molecular biology, molecular phylogeny, and molecular diagnostic approaches to the Microsporidia. In: Wittner M, Weiss L (eds) The microsporidia and microsporidiosis. American Society of Microbiology, Washington DC, pp 129–171
Wu HB, Wu YS, Wu ZH (2005) Occurrence of a new microsporidian in the abdominal cavity of Epinephelus akaara. Acta Hydrobiol 29:150–154
Zhang JY, Wu YS, Lu YS, Wang JG (2004) Advance in research of fish microsporidia. Acta Hydrobiol Sinica 28:563–568
Zhang JY, Wu YS, Wu HB, Wang JG, Li AH, Li M (2005) Humoral immune responses of the grouper Epinephelus akaara against the microsporidium Glugea epinephelusis. Dis Aquat Org 64:121–126. doi:10.3354/dao064121
Acknowledgments
This work was partially supported by Eng. António Almeida Foundation (Porto, Portugal); FCT (Lisbon, Portugal), within the scope of the PhD fellowship grant attributed to S. Rocha (SFRH/BD/92661/2013) through the program QREN-POPH/FSE; and the project no. PRG-1436-02 of King Saud University (Riyadh, Saudi Arabia). We are grateful to Dr. Paulo Oliveira, director of the Natural Park of Madeira, for permission to catch the samples of blacktail comber, from the coastal waters of the Selvagens Nature Reserve, and to the Nature Ranger, Mr. Jacques, for help with the line fishing. This work complies with the current laws of the country where it was performed. The helpful comments and suggestions of the reviewers were greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casal, G., Rocha, S., Costa, G. et al. Ultrastructural and molecular characterization of Glugea serranus n. sp., a microsporidian infecting the blacktail comber, Serranus atricauda (Teleostei: Serranidae), in the Madeira Archipelago (Portugal). Parasitol Res 115, 3963–3972 (2016). https://doi.org/10.1007/s00436-016-5162-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5162-7