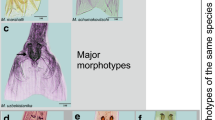
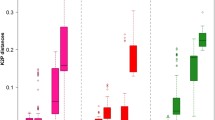
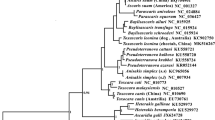
Abstract
Gastrointestinal nematodes within the subfamily Ostertagiinae (Teladorsagia, Ostertagia, and Marshallagia et al.) are among the most common infections of domesticated livestock. These parasites are of particular interest, as many of the species within this group are of economic importance worldwide. Traditionally, nematode species designations have been based on morphological criteria. However, this group possesses poorly defined species. There is an urgent need to develop a reliable technique that can distinguish species of Ostertagiinae. DNA barcoding has been proved to be a powerful tool to identify species of birds, mammals, and arthropods, but this technique has not yet been examined for identifying species of Ostertagiinae. In this study, a total of 138 mitochondrial DNA (mtDNA) cytochrome c oxidase subunit I (COI) sequences from individuals representing 11 species of Ostertagiinae were acquired by PCR for the first time. The specimens were collected from pastoral area of northern China. Genetic divergence analyses showed that mean interspecific Kimura two-parameter distances of COI (13.61 %) were about four times higher than the mean value of the intraspecific divergence (3.69 %). Then, the performance of the COI to identify species of Ostertagiinae was evaluated by identification success rates using nearest neighbor (NN) and BLASTn. The results indicated that the rates of correct sequence identification for COI were high (>80 %) when using the NN and BLASTn methods. Besides, the deep lineage divergences are detected in Teladorsagia circumcincta. Meanwhile, the analyses also detected no genetic differentiation between some species such as Ostertagia hahurica and Ostertagia buriatica. These results indicate that the traditional status of species within Ostertagiinae should be closely examined based on the molecular data.



Similar content being viewed by others
References
Bensasson D, Zhang DX, Hartl DL, Hewitt GM (2001) Mitochondrial pseudogenes: evolution’s misplaced witnesses. Trends Ecol Evol 16:314–321
Blaxter M (2003) Molecular systematics: counting angels with DNA. Nature 421:122–124. doi:10.1038/421122a
Blouin MS, Liu J, Berry RE (1999) Life cycle variation and the genetic structure of nematode populations. Heredity 83:253–259
Borji H, Raji AR, Naghibi AG (2011) The comparative morphology of Marshallagia marshalli and Ostertagia occidentalis (Nematoda: Strongylida, Trichostrongylidae) by scanning electron microscopy. Parasitol Res 108:1391–1395
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL (2009) BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi:10.1186/1471-2105-10-421
Casiraghi M et al (2004) Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. Int J Parasitol 34:191–203
Chen S et al (2010) Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS One 5, e8613. doi:10.1371/journal.pone.0008613
Criscione CD, Poulin R, Blouin MS (2005) Molecular ecology of parasites: elucidating ecological and microevolutionary processes. Mol Ecol 14:2247–2257. doi:10.1111/j.1365-294X.2005.02587.x
De Ley P et al (2005) An integrated approach to fast and informative morphological vouchering of nematodes for applications in molecular barcoding. Philos T Roy Soc B 360:1945–1958
Derycke S, Fonseca G, Vierstraete A, Vanfleteren J, Vincx M, Moens T (2008) Disentangling taxonomy within the Rhabditis (Pellioditis) marina (Nematoda, Rhabditidae) species complex using molecular and morphological tools. Zool J Linn Soc-lond 152:1–15
Derycke S, Vanaverbeke J, Rigaux A, Backeljau T, Moens T (2010) Exploring the use of cytochrome oxidase c subunit 1 (COI) for DNA barcoding of free-living marine nematodes. PLoS One 5, e13716. doi:10.1371/journal.pone.0013716
Drożdż J (1995) Polymorphism in the Ostertagiinae Lopez-Neyra, 1947 and comments on the systematics of these nematodes. Syst Parasitol 32:91–99
Elsasser SC, Floyd R, Hebert PD, Schulte-Hostedde AI (2009) Species identification of North American guinea worms (Nematoda: Dracunculus) with DNA barcoding. Mol Ecol Resour 9:707–712. doi:10.1111/j.1755-0998.2008.02393.x
Floyd R, Abebe E, Papert A, Blaxter M (2002) Molecular barcodes for soil nematode identification. Mol Ecol 11:839–850
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotech 3:294–299
Gibbons LM, Khalil LF (1982) A key for the identification of genera of the nematode family Trichostrongylidae Leiper, 1912. J Helminthol 56:185–233
Godfray HC (2002) Challenges for taxonomy. Nature 417:17–19. doi:10.1038/417017a
Goldfinch GM, Smith WD, Imrie L, Mclean K, Inglis NF, Pemberton AD (2008) The proteome of gastric lymph in normal and nematode infected sheep. Proteomics 8:1909–1918
Grillo V, Craig BH, Wimmer B, Gilleard JS (2008) Microsatellite genotyping supports the hypothesis that Teladorsagia davtiani and Teladorsagia trifurcata are morphotypes of Teladorsagia circumcincta. Mol Biochem Parasit 159:59–63. doi:10.1016/j.molbiopara.2008.01.001
Hebert PD, Gregory TR (2005) The promise of DNA barcoding for taxonomy. Syst Biol 54:852–859. doi:10.1080/10635150500354886
Herd RP (1988) Control strategies for ostertagiasis. Vet Parasitol 27:111–123
Hojun S, Buhay JE, Whiting MF, Crandall KA (2008) Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc Natl Acad Sci USA 105:13486–13491
Hugot JP, Morand S, Baujard P (2001) Biodiversity in helminths and nematodes as a field of study: an overview. Nematology 3:199–208
Hurst GD, Jiggins FM (2005) Problems with mitochondrial DNA as a marker in population, phylogeographic and phylogenetic studies: the effects of inherited symbionts. Proc Biol Sci 272:1525–1534. doi:10.1098/rspb.2005.3056
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Lancaster MB, Hong C (1981) Polymorphism in nematodes. Syst Parasitol 3:29–31
Lee IM, Hammond RW, Davis RE, Gundersen DE (1993) Universal amplification and analysis of pathogen 16S rDNA for classification and identification of mycoplasmalike organisms. Phytopathology 83:834–842
Leignel V, Cabaret J (2001) Are Teladorsagia circumcincta (Nematoda) morphs equally able to survive under anthelmintic treatment in sheep on pastures? Parasitol Res 87:687–692
Lichtenfels JR, Hoberg EP (1993) The systematics of nematodes that cause ostertagiasis in domestic and wild ruminants in North America: an update and a key to species. Vet Parasitol 46:33–53
Lv J et al (2014) Assessment of four DNA fragments (COI, 16S rDNA, ITS2, 12S rDNA) for species identification of the Ixodida (Acari: Ixodida). Parasite Vector 7:93. doi:10.1186/1756-3305-7-93
Maia VH, Mata CS, Franco LO, Cardoso MA, Cardoso SR, Hemerly AS, Ferreira PC (2012) DNA barcoding Bromeliaceae: achievements and pitfalls. PLoS One 7, e29877. doi:10.1371/journal.pone.0029877
Nejsum P, Frydenberg J, Roepstorff A, Parker ED Jr (2005) Population structure in Ascaris suum (Nematoda) among domestic swine in Denmark as measured by whole genome DNA fingerprinting. Hereditas 142:7–14. doi:10.1111/j.1601-5223.2005.01864.x
Nylander JAA (2005) MrModeltest v2. Computer program distributed by the author. Evolutionary Biology Centre, Uppsala University, Uppsala
Prosser SWJ, Velarde‐Aguilar MG, Hebert PDN (2013) Advancing nematode barcoding: a primer cocktail for the cytochrome c oxidase subunit I gene from vertebrate parasitic nematodes. Mol Ecol Resour 13:1108–1115
Ratnasingham S, Hebert PD (2007) Bold: the barcode of life data system (http://www.barcodinglife.org). Mol Ecol Notes 7:355–364 doi:10.1111/j.1471-8286.2007.01678.x
Riggio V et al (2014) A joint analysis to identify loci underlying variation in nematode resistance in three European sheep populations. J Anim Breed Genet 131:426–436. doi:10.1111/jbg.12071
Ronquist F et al (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. doi:10.1093/sysbio/sys029
Ross HA, Murugan S, Li WL (2008) Testing the reliability of genetic methods of species identification via simulation. Syst Biol 57:216–230. doi:10.1080/10635150802032990
Schindel DE, Miller SE (2005) DNA barcoding a useful tool for taxonomists. Nature 435:17. doi:10.1038/435017b
Shapiro-Ilan DI, Gaugler R (2002) Production technology for entomopathogenic nematodes and their bacterial symbionts. J Ind Microbiol Biot 28:137–146
Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN (1993) Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasit 58:283–292
Tamura K (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28
Theodoridis S, Stefanaki A, Tezcan M, Aki C, Kokkini S, Vlachonasios KE (2012) DNA barcoding in native plants of the Labiatae (Lamiaceae) family from Chios Island (Greece) and the adjacent Cesme-Karaburun Peninsula (Turkey). Mol Ecol Resour 12:620–633. doi:10.1111/j.1755-0998.2012.03129.x
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Whitworth TL, Dawson RD, Magalon H, Baudry E (2007) DNA barcoding cannot reliably identify species of the blowfly genus Protocalliphora (Diptera: Calliphoridae). Pro Biol Sci 274:1731–1739. doi:10.1098/rspb.2007.0062
Wijova M, Moravec F, Horak A, Modry D, Lukes J (2005) Phylogenetic position of Dracunculus medinensis and some related nematodes inferred from 18S rRNA. Parasitol Res 96:133–135. doi:10.1007/s00436-005-1330-x
Wu S, Zhang L, Wang F (2001) Fauna Sinica: Nematoda Rhabditida: Strongylata (I). Science Press, Beijing
Zarlenga DS, Hoberg EP, Stringfellow F, Lichtenfels JR (1998) Comparisons of two polymorphic species of Ostertagia and phylogenetic relationships within the Ostertagiinae (Nematoda: Trichostrongyloidea) inferred from ribosomal DNA repeat and mitochondrial DNA sequences. J Parasitol 84:806–812
Zhang CY, Wang FY, Yan HF, Hao G, Hu CM, Ge XJ (2012) Testing DNA barcoding in closely related groups of Lysimachia L. (Myrsinaceae). Mol Ecol Resour 12:98–108. doi:10.1111/j.1755-0998.2011.03076.x
Acknowledgments
We thank Dr. Zhang Luping (Hebei Normal University) and Dr. Yang Xiaoye (Inner Mongolia Agricultural University) for their kind assistance in morphological identification of Ostertagiinae specimens. We also thank Dr. Ji Xincheng (Xinjiang Entry-Exit Inspection and Quarantine Bureau) and Dr. Zhao Qing (Qinghai Entry-Exit Inspection and Quarantine Bureau) for their kind assistance in collection of Ostertagiinae specimens. This research was supported by the National Science and Technology Support Programs of the Ministry of Science and Technology of China (Grant Nos: 2012BAK11B04, 2013BAD12B01).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Shaoqiang Wu and Xiangmei Lin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lv, J., Zhang, Y., Feng, C. et al. Species discrimination in the subfamily Ostertagiinae of Northern China: assessment of DNA barcode in a taxonomically challenging group. Parasitol Res 115, 987–996 (2016). https://doi.org/10.1007/s00436-015-4826-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4826-z