Abstract
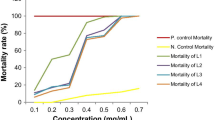
The present study was carried out to establish the properties of Carica papaya leaf extract and bacterial insecticide, spinosad on larvicidal and pupicidal activity against the chikungunya vector, Aedes aegypti. The medicinal plants were collected from the area around Bharathiar University, Coimbatore, India. C. papaya leaf was washed with tap water and shade-dried at room temperature. An electrical blender powdered the dried plant materials (leaves). The powder (500 g) of the leaf was extracted with 1.5 l of organic solvents of methanol for 8 h using a Soxhlet apparatus and then filtered. The crude leaf extracts were evaporated to dryness in a rotary vacuum evaporator. The plant extract showed larvicidal and pupicidal effects after 24 h of exposure; however, the highest larval and pupal mortality was found in the leaf extract of methanol C. papaya against the first- to fourth-instar larvae and pupae of values LC50 = I instar was 51.76 ppm, II instar was 61.87 ppm, III instar was 74.07 ppm, and IV instar was 82.18 ppm, and pupae was 440.65 ppm, respectively, and bacterial insecticide, spinosad against the first to fourth instar larvae and pupae of values LC50 = I instar was 51.76 ppm, II instar was 61.87 ppm, III instar was 74.07 ppm, and IV instar was 82.18 ppm, and pupae was 93.44 ppm, respectively. Moreover, combined treatment of values of LC50 = I instar was 55.77 ppm, II instar was 65.77 ppm, III instar was 76.36 ppm, and IV instar was 92.78 ppm, and pupae was 107.62 ppm, respectively. No mortality was observed in the control. The results that the leaves extract of C. papaya and bacterial insecticide, Spinosad is promising as good larvicidal and pupicidal properties of against chikungunya vector, A. aegypti. This is an ideal eco-friendly approach for the control of chikungunya vector, A. aegypti as target species of vector control programs.
Similar content being viewed by others
References
Aarthi N, Murugan K (2010) Larvicidal and repellent activity of Vetiveria zizanioides L, Ocimum basilicum Linn and the microbial pesticide spinosad against malarial vector, Anopheles stephensi Liston (Insecta: Diptera: Culicidae) Journal of Biopesticides 3(1) 199–204
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–266
Adebowale A, Ganesan AP, Prassad RNV (2002) Papaya (Carica papaya) consumption is unsafe in pregnancy: Fact or fable? Scientific evaluation of a common belief in some parts of Asia using a rat model. British J Nutr 88:199–203
Alder HL, Rossler EB (1977) Introduction to probability and statistics. Freeman, San Francisco, p 246
Ali A (1981) Bacillus thuringiensis serovar. israelensis (ABG-6108) against Chironomids and some non target aquatic invertebrates. J Invertebr Pathol 38:264–272
Allan SA, Kline DL (1998) Larval rearing water and preexisting eggs influence oviposition by Aedes aegypti and A. albopictus (Diptera: Culicidae). J Med Entomol 35:943–947
Amer A, Mehlhorn H (2006) Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera: Culicidae). Parasitol Res 99:466–472
Amer A, Mehlhorn H (2006a) Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera: Culicidae). Parasitol Res 99:466–472
Amer A, Mehlhorn H (2006b) Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol Res 99:478–490
Balaraman K, Balasubramanian M, Jambulingam P (1983) Field trial of Bacillus thuringiensis H-14 (VCRC B-17) against Culex and Anopheles larvae. Ind J Med Res 77:38–43
Beehler JW, Mulla MS (1993) Effect of the insect growth regulator methoprene on the ovipositional behavior of Aedes aegypti and Culex quinquefasciatus. J Am Mosq Control Assoc 9:13–16
Bond JG, Marina CF, Williams T (2004) The naturally derived insecticide spinosad is highly toxic to Aedes and Anopheles mosquito larvae. Med Vet Entomol 18:50–56
Breslin WJ, Marty MS, Vedula U, Liberacki AB, Yano BL (2000) Developmental toxicity of spinosad administered by gavage to CD rats and New Zealand white rabbits. Food Chem Toxicol 38:1103–1112
Bret BL, Larson LL, Schoonover JR, Sparks TC, Thompson GD (1997) Biological properties of Spinosad. Down to Earth 52:6–13
Cetin H, Yanikoglu A, Cilek JE (2005a) Evaluation of the naturally-derived insecticide spinosad against Culex pipiens L. (Diptera: Culicidae) larvae in septic tank water in Antalya, Turkey. J Vector Ecol 30:151–154
Cetin H, Yanikoglu A, Cilek JE (2005b) Evaluation of the naturally derived insecticide spinosad against Culex pipiens L. (Diptera: Culicidae) larvae in septic tank water in Antalya, Turkey. J Vect Ecol 30:151–154
Chowdhury N, Ghosh A, Chandra G (2008) Mosquito larvicidal activities of Solanum villosum berry extract against the dengue vector Stegomyia aegypti. BMC Complement Altern Med 8:10 (published online)
Cisneros J, Goulson D, Derwent LC, Penagos DI, Hernández O, Williams T (2002) Toxic effects of spinosad on predatory insects. Biol Control 23:156–163
Cleveland CB, Bormett GA, Saunders DG, Powers FL, McGibbon AS, Reeves GL, Rutherford L, Balcer JL (2002) Environmental fate of spinosad. 1. Dissipation and degradation in aqueous systems. J. Agric. Food Chem 50:3244–3256
Copping LG, Menn JJ (2001) Biopesticides: a review of their action, applications and efficacy. Pest Manag Sci 56:651–676
Corbet PS, Chadee DD (1993) An improved method for detecting substrate preferences shown by mosquitoes that exhibit “skip oviposition.”. Physiol Entomol 18:114–118
Curtis CF, Lines JD, Baolin L, Renz A (1991) Natural and synthetic repellents. In: Curtis CF (ed) Control of disease vectors in the community. Wolfe, London, pp 75–92
Darriet F, Corbel V (2006) Laboratory evaluation of pyriproxyfen and spinosad, alone and in combination, against Aedes aegypti larvae. J Med Entomol 43:1190–1194
Darriet F, Duchon S, Hougard JM (2005) Spinosad: a new larvicide against insecticide-resistant mosquito larvae. J Am Mosq Control Assoc 21:495–496
DeAmicis CV, Dripps JE, Hatton CJ, Karr LL (1997) Physical and biological properties of the spinosyns: novel macrolide pest-control agents from fermentation. In: Hedin PA, Hollingworth RM, Masler EP, Miyamoto J, Thompson DG (eds) Phytochemicals for pest control, Symposium Series 658. American Chemical Society, Washington, pp 144–154
Dominguez de Maria P, Sinisteraa JB, Tsai Sw, Alcantara AR (2006) Biotech Advances 24: 493–499
Emeruwa AC (1982) Antibacterial substance from Carica papaya fruit extract. J Nat Prod 45:123–127
Eswarappa S, Benjamin SPE (2007) Renal failure and neuromuscular weakness in Cleistanthus collinus poisoning. J Assoc Physicians India 55:85–86
Fay RW, Eliason DA (1966) A preferred oviposition site as a surveillance method for Aedes aegypti. Mosq News 26:531–535
Federici BA, Park HW, Bideshi DK, Wirth MC, Johnson JJ (2003) Recombinant bacteria for mosquito control. J Exp Biol 206:3877–3885
Filho ECO, Paumgartten FJ (2000) Toxicity of Euphorbia milii latex and niclosamide to snails and nontarget aquatic species. Ecotoxicol Environ Saf 46 (3):342–350
Finney DJ (1971) Probit analysis. Cambridge University Press London. pp.68–78
Garcia R, Desrochers BD (1979) Toxicity of Bacillus thuringiensis var. israelensis to some California mosquitoes under different conditions. Mosq News 39:541–544
Ghosh A, Chowdhury N, Chandra G (2008) Laboratory evaluation of a phytosteroid compound of mature leaves of day jasmine (Solanaceae: Solanales) against larvae of Culex quinquefasciatus (Diptera: Culicidae) and nontarget organisms. Parasitol Res 103:221–277
Goldberg L, Margalit J (1977) A bacterial spore demonstrating rapid larvicidal activity against Anopheles sergentii, Uranotaenia unguiculata. Culex univitattus, Aedes aegypti, and Culex pipiens. Mosq News 37:355–358
Gould F, Anderson A, Landis D, Van Mellaert J (1991) Feeding behaviour and growth of Heliothis virescens larvae on diets containing Bacillus thuringiensis formulations or endotoxins. Entomol Exp Appl 58:199–210
Govindarajan M (2009) Bioefficacy of Cassia fistula Linn. (Leguminosae) leaf extract against chikungunya vector, Aedes aegypti (Diptera: Culicidae). Eur Rev Med Pharmacol Sci 13:99–103
Gubler DJ (1998) Dengue. In: Monath TP (ed) The arboviruses epidemiology and ecology. CRC Press, Boca Raton, pp 223–260
Gubler DJ (2004) The changing epidemiology of yellow fever and dengue, 1900 to 2003: full circle? Comp Immunol Microbiol Infect Dis 27:319–330
Gubler DJ, Clark GG (1995) Dengue/dengue hemorrhaghic fever. The emergence of a global health problem. Emerg Infect Dis 1:55–57
Guha-Sapir D, Schimme B (2005) Dengue fever: new paradigms for a changing epidemiology. Emerg. Themes Epidemiol 2:1
Harrington LC, Edman JD (2001) Indirect evidence against delayed “skip-oviposition” behavior by Aedes aegypti (Diptera: Culicidae) in Thailand. J Med Entomol 38:641–645
Huet J, Looze Y, Bartik K, Raussens V, Wintjens R, Boussard P (2006) Structural characterization of the papaya cysteine protinases at low pH. Biochem Biophy Res Commun 341:620–626
Jang YS, Kim MK, Ahn YS, Lee HS (2002) Larvicidal activity of Brazilian plant against Aedes aegypti and Culex pipiens (Diptera: Culicidae) Agri Chem. Biotechnol 4:131–134
Kannathasan K, Senthilkumar A, Chandrasekaran M, Venkatesalu V (2007) Differential larvicidal efficacy of four species of Vitex against Culex quinquefasciatus larvae. Parasitol Res 101(6):1721–1723
Kirst HA, Michel KH, Mynderse JS, Chio EH, Yao RC, Nakatsukasa WM, Boech LD, Occlowitz JL, Paschal JW, Deeter JB, Thompson GD (1992) Discovery, isolation, and structure elucidation of a family of structurally unique fermentation-derived tetracyclic macrolides. In: Baker DR, Fenyes JG, Steffans JJ (eds) Synthesis and chemistry of agrochemicals III. American Chemical Society, Washington, pp 214–225
Kovendan K, Murugan K (2011) Effect of medicinal plants on the mosquito vectors from the different agro-climatic regions of Tamil Nadu, India. Adv in Environ Biol 5(2):335–344
Kovendan K, Murugan K, Vincent S, Kamalakannan S (2011a) Larvicidal efficacy of Jatropha curcas and bacterial insecticide, Bacillus thuringiensis, against lymphatic filarial vector, Culex quinquefasciatus Say. (Diptera: Culicidae). Parasitol Res. doi:10.1007/s00436-011-2368-6
Kovendan K, Murugan K, Vincent S, Donald R. Barnard (2011b) Studies on larvicidal and pupicidal activity of Leucas aspera Willd. (Lamiaceae) and bacterial insecticide, Bacillus sphaericus, against malarial vector, Anopheles stephensi Liston. (Diptera: Culicidae) Parasitol Res DOI 10.1007/s00436-011-2469-2
Kramer V (1984) Evaluation of Bacillus sphaericus and B. thuringiensis H-14 for mosquito control in rice fields. Indian J Med Res 80:642–648
Lacey LA, Lacey CM (1990) The medical importance of rice and mosquitoes and their control using alternatives to chemical insecticides. J Am Mosq Control Assoc 2:1–93
Lima MG, Maia IC, Sousa BD, Morais SM, Freitas SM (2006) Effect of stalk and leaf extracts from Euphorbiaceae species on Aedes aegypti (Diptera, Culicidae) larvae. Rev Inst Med Trop Sao Paulo 48(4):211–214
Liu S, Li QX (2004) Photolysis of spinosyns in seawater, stream water and various aqueous solutions. Chemosphere 56:1121–1127
Liu H, Cupp EW, Guo A, Liu N (2004a) Insecticide resistance in Alabama and Florida mosquito strains of Aedes albopictus. J Med Entomol 41:946–952
Liu H, Cupp EW, Micher KM, Guo A, Liu N (2004b) Insecticide resistance and cross-resistance in Alabama and Florida strains of Culex quinquefaciatus. J Med Entomol 41:408–413
Ludlum CT, Felton G, Duffey SS (1991) Plant defense: chlorogenic acid and polyphenol oxidase enhance toxicity of Bacillus thruringiensis subsp. kurstaki to Heliothis zea. J Chem Ecol 17:217–237
Maheswaran R, Sathis S, Ignacimuthu S (2008) Larvicidal activity of Leucus aspera (Willd.) against the larvae of Culex quinquefasciatus Say. and Aedes aegypti L. Int Journal of Int Biol 2(3):214–217
Mather TN, DeFoliart GR (1983) Repellency and initial toxicity of Abate and Dursban formulations to Aedes triseriatus in oviposition sites. Mosq News 43:474–479
Mehlhorn H, Schmahl G, Schmidt J (2005) Extract of the seeds of the plant Vitex agnus castus proven to be highly efficacious as a repellent against ticks, fleas, mosquitoes and biting flies. Parasitol Res 95(5):363–365
Mello VJ, Gomes MT, Lemos FO, Delfino JL, Andrade SP, Lopes MT, Salas CE (2008) The gastric ulcer protective and healing role of cysteine proteinases from Carica candamarcensis. Phytomedicine 15:237–244
Moore CG (1977) Insecticide avoidance by ovipositing Aedes aegypti. Mosq News 37:291–293
Morena-Sanchez R, Hayden M, James C (2006) A web based multimedia spatial information sytem to document Aedes aegypti breeding sites and dengue fever risk along the US-Mexico border. Health Place 12:715–727
Muller G, Schlein Y (2006) Sugar questing mosquitoes in arid areas gather on scarce blossoms that can be used for control. Int J Parasitol 36:1077–1080
Munoz V, Sauvain M, Bourdy G, Callapa J, Rojas I, Vargas L, Tae A, Deharo E (2000) The search for natural bioactive compounds through a multidisciplinary approach in Bolivia Part II. Antimalarial activity of some plants used by Mosetene indians. J Ethnopharmacol 69:139–155
Okeniyi JA, Ogunlesi TA, Oyelami OA, Adeyemi LA (2007) Effectiveness of dried Carica papaya seeds against human intestinal parasitosis: a pilot study. J Med Food 10:493–499
Olagunju JA, Ogunlana CO, Gbile Z (1995) Preliminary studies on the hypoglycemic activity of ethanolic extract of unripe, mature fruits of pawpaw. Nig J Biochem Mol Biol 10:21–23
Pancharoen C, Kulwichit W, Tantwichien T, Thisyakorn U, Thisyakorin C (2002) Dengue infection: global concern. J Med Assoc Thai 85:25–33
Pates H, Curtis C (2005) Mosquito behavior and vector control. Annu Rev Entomol 50:53–70
Perez CM, Marina CF, Bond JG, Rojas JC, Valle J, Williams T (2007) Spinosad, a naturally derived insecticide, for control of Aedes aegypti (Diptera: Culicidae): efficacy, persistence, and elicited oviposition response. J Med Entomol 44(4):631–638
Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K (2008) Isolation and identification of mosquito larvicidal compound from Abutilon indicum (Linn.) Sweet. Parasitol Res 102:981–988
Rahuman AA, Bagavan A, Kamaraj C, Saravanan E, Zahir AA, Elango G (2009a) Efficacy of larvicidal botanical extracts against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 104(6):1365–1372
Rawani A, Haldar KM, Ghosh A, Chandra G (2009) Larvicidal activities of three plants against filarial vector Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 105:1411–1417
Roark RC (1947) Some promising insecticidal plants. Econ Bot 1:437–445
Romi R, Proietti S, Di Luca M, Cristofaro M (2006) Laboratory evaluation of the bioinsecticide spinosad for mosquito control. J Am Mosq Control Assoc 22:93–96
Salgado VL (1997) The mode of action of spinosad and other insect control products. Down to Earth 52:35–44
Salgado VL (1998) Studies on the mode of action of spinosad: insect symptoms and physiology correlates. Pest Biochem Physiol 60:91–102
Sarathchandra G, Balakrishnamoorthy P (1998) Acute toxicity of Cleistanthus collinus, an indigenous poisonous plant in Cavia porcellus. J Environ Biol 1:145–148
Saunders DG, Bret BL (1997) Fate of spinosad in the environment. Down to Earth 52:14–20
Seigler DS, Pauli GF, Nahrstedt A, Leen R (2002) Cyanogenic allosides and glucosides from Passiflora edulis and Carica papaya. Phytochemistry 60:873–882
Shaalan EAS, Canyonb D, Younesc MWF, Abdel-Wahaba H, Mansoura AH (2005) A review of botanical phytochemicals with mosquitocidal potential. Environ Int 31:1149–1166
Sharma P, Mohan L, Srivastava CN (2005) Larvicidal potential of Nerium indicum and Thuja oriertelis extracts against malaria and Japanese encephalitis vector. J Environ Biol 26(4):657–660
Snyder DE, Meyer J, Zimmermann AG, Qiao M, Gissendanner SJ, Cruthers LR, Slone RL, Young DR (2007) Preliminary studies on the effectiveness of the novel pulicide, spinosad, for the treatment and control of fleas on dogs. Vet Parasitol 150(4):345–351
Sukumar K, Perich MJ, Boobar LR (1991) Botanical derivatives in mosquito control: a review. J Am Mosq Control Assoc 7:210–237
Thomas TG, Raghavendra K, Lal S, Saxena VK (2004) Mosquito larvicidal properties of latex from unripe fruits of Carica papaya Linn. (Caricaceae). J Commun Dis 36:290–292
Thompson DG, Harris BJ, Buscarini TM, Chartrand DT (2002) Fate of spinosad in litter and soils of a white spruce plantation in central Ontario. Pest Manag Sci 58:397–404
Tripathi YC, Rathore M (2001) Role of lipids in natural defense and plant protection. Indian J Forestry 24:448–455
Van den Berghe G (1978) Curr Top Cell Regul 13:97–135
Verma KVS (1986) Deterrent effect of synthetic pyrethroids on the oviposition of mosquitoes. Curr Sci 55:373–375
Vezzani D, Rubio A, Velazquez SM, Schweigmann N, Wiegand T (2005) Detailed assessment of microhabitat suitability for Aedes aegypti (Diptera: Culicidae) in Buenos Aires, Argentina. Acta Trop 95:123–131
Watson GB (2001) Actions of insecticidal spinosyns on caminobutyric acid responses from small-diameter cockroach neurons. Pest Biochem Physiol 71:20–28
WHO (1998) Dengue hemorrhagic fever. Diagnosis, treatment, prevention and control. World Health Organization, Ginebra, Suiza
Williams T, Valle J, Vinuela E (2003) Is the naturally derived insecticide spinosad compatible with insect natural enemies? Biocontrol Sci Technol 13:459–475
Zolotar RM, Bykhovets AI, Kashkan ZN, Chernov YG, Kovganko NV (2002) Structure-activity relationship for insecticidal steroids. VI. 5, 6-Disubstituted b-sitosterols. Chem Nat Compd 38(2):167–170
Acknowledgments
The authors are thankful to the Department of Science and Technology (DST), New Delhi, India and Tamil Nadu State Council for Science and Technology (TNSCST), Chennai, Tamil Nadu for providing financial support for the present work. The authors are grateful to Dr. K. Sasikala, Professor and Head, Department of Zoology, Bharathiar University for the laboratory facilities provided.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kovendan, K., Murugan, K., Naresh Kumar, A. et al. Bioefficacy of larvicdial and pupicidal properties of Carica papaya (Caricaceae) leaf extract and bacterial insecticide, spinosad, against chikungunya vector, Aedes aegypti (Diptera: Culicidae). Parasitol Res 110, 669–678 (2012). https://doi.org/10.1007/s00436-011-2540-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2540-z