Abstract
Neospora caninum is a protozoan parasite which causes abortion in cattle as well as reproduction problems and neurological disorders in dogs. To assess the prevalence of the parasite in urban dogs in the Mazovian Voivodeship, Central Poland, serum samples from 257 dogs were analyzed for the presence of specific IgG antibodies. The examined dogs visited three private veterinary clinics located in Warsaw due to control tests, vaccinations, or other reasons not directly connected with neosporosis. Using ELISA and Western blot, antibodies against the parasite were detected in 56 out of 257 dogs, giving a prevalence of 21.7%. A greater prevalence was observed in female dogs than in males, 28% and 17.3%, respectively, and the differences were statistically significant (p < 0.05). There were no significant differences in seroprevalence of Neospora infection within the age groups (p > 0.05). This study indicates the presence of N. caninum in the Mazovian Voivodeship, in dogs which live in urban areas and exposure of these dogs to the parasite. The fact that seropositive dogs had no contact with cattle confirms the important role of dogs in the parasite’s epidemiology.
Similar content being viewed by others
Introduction
Neospora caninum is an intracellular protozoan parasite that provokes neurological disorders, recurrent abortion, and neonatal mortality in dogs. The parasite has a wide range of intermediate hosts, including cows, sheep, goats, horses, bison, and deer.
Neosporosis is especially important in cattle; the parasite has been recognized as one of the main causes of abortion in dairy cattle worldwide (Dubey 2003). Dogs and coyotes are the only definitive hosts of N. caninum that have been described to date, but possibly other carnivores such as foxes and wolves can act as definitive hosts for the parasite. Dogs are both intermediate and definitive hosts of N. caninum and play a crucial role in horizontal transmission of this protozoan to other animals (McAllister et al. 1998; Gondim et al. 2004).
In the dog population, neosporosis is spread by vertical infection from bitch to offspring and/or horizontal infection through ingestion of infected tissues of bovine origin. The parasite may be transmitted to cattle through the ingestion of oocysts that are shed in the feces of acutely infected dogs or by congenital infection from mother to fetus via the placenta. Although N. caninum is transplacentally transmitted very efficiently in cattle and oocysts are rarely found in dog feces, dogs are considered essential in the life cycle of this parasite (Schares et al. 2005; Dubey et al. 2007).
The diagnosis of neosporosis in dogs is difficult due to nonspecific clinical signs. The clinical symptoms depend on the placement of parasites. The parasite can reach different organs, mainly the central nervous system, the brain and spinal cord, but also muscles, heart, liver, kidneys and skin, where it can form cysts and persist for a long time leading to chronic disease. In affected dogs, the most common symptoms include progressive paralysis of the hind limbs, difficulty in swallowing, paralysis of the jaw, muscle flaccidity and muscle atrophy, or even heart failure. A cutaneous disease or diarrhea in case of digestive neosporosis may also occur. In general, neosporosis can be asymptomatic in adult dogs and the most severe cases of disease occur in young, congenitally infected puppies (Dubey and Lindsay 1996; Dubey 2003).
Serological methods such as the indirect fluorescent antibody test (IFA), Neospora agglutination test, enzyme-linked immunosorbent assay (ELISA), and Western blot can be used for the detection of specific antibodies in sera (Hemphill and Gottstein 2000; Dubey and Schares 2006; Dubey et al. 2007). Using serological methods N. caninum infections in dogs have been reported in many parts of the world. In Europe, the prevalence rates of canine neosporosis varied between 0.5% and 28.9% in different countries (Dubey et al. 2007).
In Poland, the presence of N. caninum has been previously confirmed serologically in aborting cows and later in other intermediate hosts such as bison and red deer (Cabaj et al. 2000; Cabaj et al. 2005; Goździk et al. 2010). Moreover, the antibodies against N. caninum were detected in definitive hosts such as dogs and foxes in southwestern Poland. Using a commercially available IFA test, the antibodies against the parasite were detected in low titres in two of 45 red foxes and in one out of 60 farmed foxes (Śmielewska-Łoś et al. 2003) and in 18 out of 110 tested dogs (Płoneczka and Mazurkiewicz 2008). Neospora infection was also serologically confirmed in ten of 29 farm dogs living in close contact with dairy cattle herds in the eastern part of Poland, giving a prevalence of 34.5% (Goździk et al. 2009). Nevertheless, there is still little information about the presence of N. caninum in definitive hosts in other parts of Poland, possible transmission routes of the parasite and the rate of infection among dogs. The aim of this study was to assess the prevalence of N. caninum in dogs which live in urban areas in Mazovia Voivodeship, Central Poland and have no contact with cattle.
Materials and methods
Sampling of dogs
Blood samples were collected between March 2008 and May 2009. Samples were obtained from 257 randomly chosen dogs during clinical examination in three private veterinary clinics located in Warsaw. A blood sample was drawn from the cephalic vein of each dog with a 20-gauge needle to a blood collection tube. The blood was centrifuged at 1,000 × g for 15 min, the sera collected and stored at −20°C until analyzed. Epidemiological information about the sex and age was recorded and the general clinical status of the dogs was evaluated. Dogs were divided into the following four age groups: under 1 year old, 1 to 5, 5 to 10, and over 10 years of age.
Serological examination
ELISA
The presence of antibodies to N. caninum was demonstrated by ELISA modified from Björkman et al. (1994a). In this assay, crude antigen from tachyzoites of the Polish isolate, Nc-PolB1 (Goździk and Cabaj 2007) was used as a capture antigen in the final dilution of 5 μg/ml. Horseradish peroxidase conjugated anti-dog IgG (Bethyl Laboratories, Inc.) diluted 1:20,000 in phosphate-buffered saline (PBS) was used as the secondary antibody. All sera were diluted 1:100 in PBS with 0.05% Tween 20 and analyzed in duplicate.
Since no positive and negative control was available and the crude antigen based ELISA was not validated, all sera with an optical density value exceeding 0.200 absorbance units were additionally analyzed by Western blot as a reference method. The cutoff value of 0.200 was chosen arbitrarily in this study and was based on the validation of the immunostimulating complex enzyme-linked immunoassay (iscom-ELISA) against an indirect fluorescent antibody test (IFAT) using cattle sera (Frössling et al. 2003).
Electrophoresis and Western blot analysis
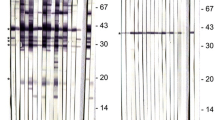
SDS-polyacrylamide gel electrophoresis and Western blot analysis were performed essentially as described by Björkman et al. (1994a) and Björkman and Hemphil (1998). For analysis under reducing conditions, 2X Laemmli sample buffer containing 10% beta-mercaptoethanol (Sigma) was added to the antigen preparation and soluble proteins were denatured by boiling the mixture for 5 min. The samples were electrophoresed on a 5% stacking gel and 12% resolving gel and the separated polypeptides were transferred to nitrocellulose membranes (pore size, 0.2 μm; Bio-Rad Laboratories). Membranes were blocked for 1 h in Tris-buffered saline (20 mM Tris, 0.9% NaCl, pH 9.0) containing 5% non-fat dry milk. The blocked membranes were cut into 3-mm-wide strips. Then the blotted polypeptides were exposed to the examined sera diluted 1:50 in PBS–Tween 20 buffer containing 5% non-fat dry milk for 1 h at room temperature. Peroxidase-labeled goat anti-dog IgG-heavy and light chain (Bethyl Laboratories, Inc.) diluted 1:5,000 in PBS–Tween was used as the secondary antibody and the membranes were incubated for 1 h at 37°C. Immunoreactive proteins were detected with DAB (3′3-diaminobenzidine tetrahydrochloride) (Sigma). A serum was regarded as positive when it reacted with at least two of the five immunodominant Neospora-specific antigens (55–53, 48–47, 37–35, 27, and 18–16 kDa) (Björkman et al. 2007).
Statistical analysis
Differences in seroprevalence between sex and age groups were analyzed using the chi-square test at P ≤ 0.05 of significance level. The calculations were performed using the STATISTICA Software (Series 1203b, version 6.1 for Windows, StatSoft, Inc.).
Results
A total of 257 dog sera were tested using serological methods, ELISA based on crude tachyzoite antigen and Western blot. The OD values of the analyzed dog sera obtained in ELISA varied between 0.133 and 3.126. The OD values of the background (well coated with antigen but without serum) varied between 0.047 and 0.075. One hundred sixty-four sera had an OD value exceeding 0.200 and were further examined by Western blot. A serum was classified as positive when it reacted with at least two of the five immunodominant antigens (55–53, 48–47, 37–35, 27, and 18–16 kDa). The strongest reaction occurred with two major proteins characterized by a molecular weight of approximately 37–35 and 18–16 kDa, additionally an antigen of 27 kDa was also observed.
Using the Western blot confirmation test, the presence of specific anti-Neospora antibodies was detected in 56 sera and those sera, deemed N. caninum positive, gave a seroprevalence of 21.7%. The prevalence of anti-Neospora antibodies was greater among females than males (28% and 17.3%, respectively) and this difference was statistically significant (P < 0.05) (Table 1).
Four age groups were considered in the study: less than 1 year old, more than 1 year to 4 years old, more than five to ten, and more than 10 years of age. The prevalence of antibodies within the age groups varied between 8% and 27%; however, this variation was non-significant (P = 0.1733) (Table 2).
Twelve dogs, serologically classified as positive, presented clinical signs of neurological disorders attributable to neosporosis, such as epilepsy, imbalance, tremor, and ataxia. In one case dermatitis was diagnosed, and in five dogs hepatic encephalopathy was recognized. The remaining dogs were not further examined and the causative role of N. caninum was not proven.
Discussion
Out of the 257 dog serum samples tested in this study, 56 were classified as seropositive for N. caninum giving a seroprevalence of 21.7%. The detection of antibodies was carried out using an ELISA test, where the soluble protein fraction from the Polish isolate (PolB1) was used as the capture antigen. Different diagnostic tests are used to detect antibodies against Neospora in dogs.
Currently, the anti-Neospora antibodies are commonly detected using an IFA (Hemphill and Gottstein 2000). There are several companies producing ready to use slides for diagnosis of neosporosis in dogs (for example, VMRD, Inc., USA; Mega Screen FLUONEOSPORA Mega Cor, Austria; and Fuller Laboratories, USA). Commercially available IFA tests are highly specific and sensitive; nevertheless, the method is time consuming when analyzing large numbers of sera and manually reading IFA results depend on the subjective assessment of the lab worker.
Several ELISA based on a water soluble fraction of sonicated tachyzoites have been validated for use in cattle, sheep, and goats. These tests were shown to have a high diagnostic sensitivity and specificity when evaluated against an IFAT (Hemphill and Gottstein 2000); however, all serological tests require species-specific conjugates to detect specific antibodies and must be individually evaluated using known positive and negative controls and a bank of sera of known status. Our ELISA had not been validated for dog sera due to lack of an appropriate control sera (true positive and true negative). The cutoff value of 0.200 was chosen arbitrarily and was based on the validation of another ELISA, iscom-ELISA, against an IFA using cattle sera (Frössling et al. 2003). The ELISA test was used here for epidemiological studies to screen dogs from the Voivodeship to assess infection status in animals in this area.
It is worth mentioning that potential cross reactions might occur in serological assays due to the fact that N. caninum is closely related to Toxoplasma gondii. Some authors demonstrated the cross-reactivity between antigens from these parasites while using the soluble protein fraction as the capture antigen in ELISA (Nishikawa et al. 2002). The reliability of the serological results depends on the diagnostic method used in the survey. The ELISA method chosen in this study is based on the soluble protein fraction from the whole tachyzoites. Nevertheless, to eliminate false positive results and/or to confirm the presence of antibodies against Neospora, Western blot was used additionally as a confirmation method (Björkman et al. 2007). In Western blot analysis, the main immunodominant bands localized at 55–53, 48–47, 37–35, 27 and 16–18 kDa, which is in agreement with results obtained by other authors (Schares et al. 2001; Staubli et al. 2006). Based on the results obtained in the two tests, the dog sera were classified as positive.
The prevalence of 21.7% of antibodies among urban dogs obtained in this study indicates a higher rate of infection than in other European countries, like Austria 2.1% (Wanha et al. 2005), Hungary 1% (Hornok et al. 2006), Spain 2.9% (Collantes-Fernandez et al. 2008), the Czech Republic 2.6% (Vaclavek et al. 2007), or 0.5% from Sweden, the lowest seroprevalence reported in Europe (Björkman et al. 1994b).
Prevalence rates comparable to our results were found in urban dogs in northwest Italy (20.2%) (Ferroglio et al. 2007), in Catalonia (northeastern Spain) (12.2%) (Ortuno et al. 2002), in Denmark (15.3%) (Rasmussen and Jensen 1996), and in southwestern Poland, where 15% of the dogs tested showed positive results (Płoneczka and Mazurkiewicz 2008).
Differences in prevalence rates observed between the gender groups were statistically significant (P < 0.05), the prevalence of antibodies was higher in female dogs than in male dogs; however, no significant differences in neosporosis seroprevalence were found among the different age groups. The examined dogs visited veterinary clinics due to control tests, vaccinations, or other reasons not directly connected with neosporosis. Among the 56 seropositive dogs, only 12 had clinical symptoms compatible with neosporosis, which suggests that a large number of the infections occur in a subclinical form; however, these dogs were not further examined and the causative role of N. caninum had not been shown.
In Poland, neosporosis was earlier considered an issue only in dairy cattle production. Previous studies carried out on farms in the northeast of Poland showed a seroprevalence of 15.6% in aborting cows and 92.5% in offspring from seropositive dams (Cabaj et al. 2000; Moskwa and Cabaj 2003). In several dairy cattle farms in the Mazovian Voivodeship, the seroprevalence varied from 1.5% to 23% (Moskwa et al. 2005). Unfortunately, during those investigations the dogs living in close contact with infected cows were not included into the studies.
The latest data revealed a much higher seroprevalence, up to 70% in some herds in the eastern part of the country (Goździk et al. 2009). Parallel studies performed on dogs showed a prevalence of 34.5% in farm dogs living in this area. The results of serological investigations obtained in this study indicate that the examined dogs had contact with the parasite, although they lived in urban areas and showed that neosporosis is a common infection even in such dogs. It has been shown that dogs living in the countryside have a significantly greater risk of N. caninum infection than dogs living in large cities since they have direct access to parasite cysts present in placentas, fetuses, or tissues from infected livestock (Dijkstra et al. 2002; Dubey 2003).
The detection of specific antibodies in dog sera indicates the potential contact of these animals with the pathogen but is not necessarily correlated with shedding of oocysts nor related with a risk of horizontal transmission or environmental contamination, since oocysts are rarely found in dog feces (Slapeta et al. 2002; Schares et al. 2005). It is known that dogs can become infected by ingesting infected tissues, but whether they can be infected by the ingestion of oocysts is unknown (Dubey et al. 2007). It has been described that subclinically infected bitches can vertically transmit the parasitosis, and successive litters from the same bitch may be born infected. Nevertheless, vertical transmission alone could not maintain the parasite in dogs (Barber and Trees 1998).
Another explanation for the presence of antibodies in dog sera could be feeding habits, like eating raw meat containing parasite cysts. It has been reported that the consumption of raw beef can be a risk factor for N. caninum infection (Kramer et al. 2004; Dubey et al. 2007) and it is known that dogs are sometimes fed raw or poorly cooked meat by their owners. These facts could explain why in our study a seroprevalence of more than 20% in urban dogs was found.
The presence of infected dogs in a particular area should be considered as a potential risk factor of Neospora infection in cattle. It has been proven that the parasite is transmitted via placenta in cattle but also that the presence of definitive hosts is crucial for the life cycle of the parasite (Dubey 2003; Dubey et al. 2007). The results of these serological studies need to be confirmed by direct methods such as demonstration of the parasites by histopathology or PCR.
Further investigations on dog populations in Poland are needed in order to determine the possible route of infection in dogs and assess the seroprevalence of neosporosis in definitive hosts in other parts of the country.
References
Barber JS, Trees AJ (1998) Naturally occurring vertical transmission of Neospora caninum in dogs. Int J Parasitol 28:57–64
Björkman C, Hemphil A (1998) Characterization of Neospora caninum iscom antigens using monoclonal antibodies. Parasite Immunol 20:73–80
Björkman C, Lunden A, Holmdahl J, Barber J, Trees AJ, Uggla A (1994a) Neospora caninum in dogs: detection of antibodies by ELISA using an iscom antigen. Parasite Immunol 16:643–648
Björkman C, Lunden A, Uggla A (1994b) Prevalence of antibodies to Neospora cninum and Toxoplasma gondii in Swedish dogs. Acta Vet Scand 35:445–447
Björkman C, Sager H, Schares G (2007) Serology. In: Ortega-Mora LM, Gottstein B, Conraths FJ, Buxton D (eds) Protozoal abortion in farm ruminants. Guidelines for diagnosis and control. CAB International, Wallingford, pp 63–75
Cabaj W, Choromanski L, Rodgers S, Moskwa B, Malczewski A (2000) Neospora caninum infections in aborting dairy cows in Poland. Acta Parasitol 45:113–114
Cabaj W, Moskwa B, Pastusiak K, Gill J (2005) Antibodies to Neospora caninum in the blood of European bison (Bison bonasus bonasus L.) living in Poland. Vet Parasitol 128:163–168
Collantes-Fernandez E, Gomez-Bautista M, Miro G, Alvarez-Garcia G, Pereira-Bueno J, Frisuelos C, Ortega-Mora LM (2008) Seroprevalence and risk factors associated with Neospora caninum infection in different dog populations in Spain. Vet Parasitol 152:148–151
Dijkstra Th, Barkema HW, Eysker M, Hesselink JW, Wouda W (2002) Natural transmission routes of Neospora caninum between farm dogs and cattle. Vet Parasitol 105:99–104
Dubey JP (2003) Review of Neospora caninum and neosporosis in animals. Korean J Parasitol 41:1–16
Dubey JP, Lindsay DS (1996) A review of Neospora caninum and neosporosis. Vet Parasitol 67:1–59
Dubey JP, Schares G (2006) Diagnosis of bovine neosporosis. Vet Parasitol 140:1–34
Dubey JP, Schares G, Ortega-Mora LM (2007) Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev 20:323–367
Ferroglio E, Pasino M, Ronco F, Bena A, Trisciuoglio A (2007) Seroprevalence of antibodies to Neospora caninum in urban and rural dogs in north-west Italy. Zoonoses Public Health 54:135–139
Frössling J, Bonnett B, Lindberg A, Björkman C (2003) Validation of a Neospora caninum iscom ELISA without a gold standard. Prev Vet Med 57:141–153
Gondim LFP, McAllister MM, Pitt WC, Zemliecka DE (2004) Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int J Parasitol 34:159–161
Goździk K, Cabaj W (2007) Characterisation of the first Polish isolate of Neospora caninum from cattle. Acta Parasitol 52:295–297
Goździk K, Grono K, Bień J, Kozak M, Cabaj W (2009) The first evidence of neosporosis in farm dogs in Eastern Poland. World Association for the Advancement of Veterinary Parasitology 2009 Abstract Volume: 158
Goździk K, Jakubek E-B, Björkman C, Bień J, Moskwa B, Cabaj W (2010) Seroprevalence of Neospora caninum in free living and farmed red deer (Cervus elaphus) in Poland. Pol J Vet Sci 13(1):117–120
Hemphill A, Gottstein B (2000) A European perspective on Neospora caninum. Int J Parasitol 30:877–924
Hornok S, Edelhofer R, Fok E, Berta K, Fejes P, Repasi A, Farkas R (2006) Canine neosporosis in Hungary: screening for seroconversion of household, herding and stray dogs. Vet Parasitol 137:197–201
Kramer L, De Risio L, Tranquillo VM, Magnino S, Genchi C (2004) Analysis of risk factors associated with seropositivity to Neospora caninum in dogs. Vet Rec 154:692–693
McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM (1998) Dogs are definitive hosts of Neospora caninum. Int J Parasitol 28:1473–1478
Moskwa B, Cabaj W (2003) Neospora caninum: a newly recognized agent causing spontaneous abortion in Polish cattle. Med Wet 59:23–26
Moskwa B, Cabaj W, Pastusiak K, Bień J (2005) Current studies on neosporosis in Poland. Wiad Parazytol 51:65–67
Nishikawa Y, Claveria FG, Fujisaki K, Nagasawa H (2002) Studies on serological cross-reaction of Neospora caninum with Toxoplasma gondii and Hammondia heydorni. J Vet Med Sci 64:161–164
Ortuno A, Castella J, Almeria S (2002) Seroprevalence of antibodies to Neospora caninum in dogs from Spain. J Parasitol 88:1263–1266
Płoneczka K, Mazurkiewicz M (2008) Seroprevalence of Neospora caninum in dogs in southwestern Poland. Vet Parasitol 153:168–171
Rasmussen K, Jensen AL (1996) Some epidemiologic features of canine neosporosis in Denmark. Vet Parasitol 62:345–349
Schares G, Wenzel U, Müller T, Conraths FJ (2001) Serological evidence for naturally occurring transmission of Neospora caninum among foxes (Vulpes vulpes). Int J Parasitol 31:418–423
Schares G, Pantchev N, Barutzki D, Heydorn AO, Bauer C, Conraths FJ (2005) Oocysts of Neospora caninum, Hammondia heydorni, Toxoplasma gondii and Hammondia hammondi in feaces collected from dogs in Germany. Int J Parasitol 35:1525–1537
Slapeta JR, Modry D, Kyselova I, Horejs R, Lukes J, Koudela B (2002) Dog shedding oocysts of Neospora caninum: PCR diagnosis and molecular phylogenetic approach. Vet Parasitol 109:157–167
Staubli D, Nunez S, Sager H, Schares G, Gottstein B (2006) Neospora caninum immunoblotting improves serodiagnosis of bovine neosporosis. Parasitol Res 99:648–658
Śmielewska-Łoś E, Pacoń J, Jańczak M, Płoneczka K (2003) Prevalence of antibodies to Toxoplasma gondii and Neospora caninum in wildlife and farmed foxes (Vulpes vulpes). Electron J Pol Agric Univ Vet Med 6:2
Vaclavek P, Sedlak K, Hurkova L, Vodrazka P, Sebesta R, Koudela B (2007) Serological survey of Neospora caninum in dogs in the Czech Republic and a long-term study of dynamics of antibodies. Vet Parasitol 143:35–41
Wanha K, Edelhofer R, Gabler-Eduardo C, Prosl H (2005) Prevalence of antibodies against Neospora caninum and Toxoplasma gondii in dogs and foxes in Austria. Vet Parasitol 128:189–193
Acknowledgement
This work was financially supported by grant no. N308 016 31/0701 of the State Committee for Scientific Research in Warsaw, Poland.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Goździk, K., Wrzesień, R., Wielgosz-Ostolska, A. et al. Prevalence of antibodies against Neospora caninum in dogs from urban areas in Central Poland. Parasitol Res 108, 991–996 (2011). https://doi.org/10.1007/s00436-010-2143-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2143-0