Abstract
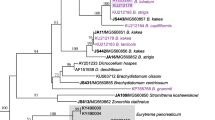
Partial large subunit (28S) rRNA gene (LSU) sequences were studied from Tentacularia coryphaenae (Cestoda: Trypanorhyncha) plerocercoids from the southern Java coast, Indonesia, collected from two different localities and five different host species. The teleost hosts belonged to four fish families with an overlapping depth range of 0–885 m. The LSU sequences were identical, demonstrating that all specimens belonged to the same species. They also corresponded to a sequence of T. coryphaenae from the Blue shark Prionace glauca in the North Atlantic, giving genetic evidence for the cosmopolitan distribution of the species. A 1,851 bp region of mitochondrial (mt) DNA (coding for partial cytochrome c oxidase subunit I (cox1), complete trnT and partial 16S ribosomal RNA) showed a very low level of intra-specific variation of 1%. Pairwise comparisons of published sequences for partial LSU rDNA and the same region of mtDNA demonstrated that the same regions varied by 8% in the mtDNA for two genotypes (G1 and G4) of Echinococcus granulosus (order Cyclophyllidea), at 16% in newly sequenced Kotorella pronosoma from the same trypanorhynch family and at 23% in Grillotia pristiophori from a different superfamily. The high genetic homogeneity in T. coryphaenae is explained by a constant gene flow between different regions and hosts along the Indonesian coast caused by extensive migrations of the second intermediate/paratenic and also the final hosts. Implications for the zoogeographical distribution, host specificity of the species and future research are discussed.

Similar content being viewed by others
References
Abe N, Ohya N, Yanagiguchi R (2005) Molecular characterization of Anisakis pegreffii larvae in Pacific cod in Japan. J Helminthol 79:303–306
Beveridge I, Campbell RA (2005) Three new genera of trypanorhynch cestodes from Australian elasmobranch fishes. Syst Parasitol 60:211–224
Beveridge I, Justine JL (2006) Gilquiniid cestodes (Trypanorhyncha) from elasmobranch fishes off New Caledonia with descriptions of two new genera and a new species. Syst Parasitol 65:235–249
Bray RA (1987) A revision of the family Zoogonidae Odhner, 1902 (Platyhelminthes: Digenea): subfamily Lepidophyllinae and comments on some aspects of biology. Syst Parasitol 9:83–123
Campbell RA, Beveridge I (1994) Order Trypanorhyncha Diesing, 1863. In: Khalil LF, Jones A, Bray RA (eds) Keys to the cestode parasites of vertebrates. CAB International, Wallingford, pp 51–148
Euzet L, Combes C (1980) Les problèmes de l’espèce chez les animaux parasites. In: Bocquet C, Génermont J, Lamotte M (eds) Les problèmes de l’espèce dans les règne animale. Mémoires de la Société Zoologique Française 40:239–285
Froese R, Pauly D (eds) (2006) Fishbase. World Wide Web electronic publication. http://www.fishbase.org, version (05/2006)
Jakob E, Palm HW (2006) Parasites of commercially important fish species from the southern Java coast, Indonesia, including the distribution pattern of trypanorhynch cestodes. Verhandlungen der Gesellschaft für Ichthyologie 5:165–191
Maddison WP, Maddison DR (1992) MacClade: analysis of phylogeny and character evolution. Sinauer Associates, Sunderland, Massachusetts
Marques JF, Cabral HN, Busi M, D’Amelio S (2006) Molecular identification of Anisakis species from Pleuronectiformes off the Portuguese coast. J Helminthol 80:47–51
Mattiucci S, Nascetti G, Clanchi R, Paggi L, Arduino P, Margolis L, Brattey J, Webb SC, D’Amelio S, Orecchia P, Bullini L (1997) Genetic and ecological data on the Anisakis simplex complex with evidence for a new species (Nematoda, Ascaridoidea, Anisakidae). J Parasitol 83:401–416
Olson PD, Littlewood DTJ, Bray RA, Mariaux J (2001) Interrelationships and evolution of the tapeworms (Platyhelminthes: Cestoda). Mol Phylogenet Evol 19:443–467
Palm HW (2004) The Trypanorhyncha Diesing, 1863. PKSPL-IPB Press, Bogor, x+710 pp
Palm HW, Klimpel S (2007a) Evolution of parasitic life in the ocean. Trends Parasitol (in press)
Palm HW, Klimpel S (2007b) Metazoan fish parasites of Macrourus berglax Lacepède, 1801 and other rattails in the North Atlantic: exploration of the deep-sea from the continental shelf. Deep Sea Res II (in press)
Rosas-Valdez R, Choudhury A, de Leon GP (2004) Phylogenetic analysis of Corallobothriinae (Cestoda: Proteocephalidea) from north American ictalurid fishes, using partial sequences of the 28S ribosomal gene. J Parasitol 90:1123–1127
Sin FYT, Waterman PB, Blair D (1992) Morphological and enzyme variations of marine tapeworms, Hepatoxylon trichiuri (Cestoda: Trypanorhyncha) and H. megacephalum, off Kaikoura coast, New Zealand. J Nat Hist 26:465–477
Swofford DL (2002) PAUP* 4.0: Phylogenetic Analysis Using Parsimony (*and other methods). Sinauer Associates, Sunderland, MA
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tkach VV, Littlewood DTJ, Olson PD, Kinsella JM, Swiderski Z (2003) Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst Parasitol 56:1–15
Van der Auwera G, Chapelle S, De Wachter R (1994) Structure of the large ribosomal subunit RNA of Phytophthora megasperma, and phylogeny of the oomycetes. FEBS Lett 338:133–136
Acknowledgements
We acknowledge the Natural History Museum London for hosting the first author. We thank Julia Llewellyn-Hughes and Claire Griffin for providing expert technical assistance with the sequencer. We acknowledge Prof. R. Overstreet, University of Mississippi, USA and Dr. I. Beveridge, University of Melbourne, Australia for collecting the reference trypanorhynch material. The study was financially supported by the German Research Council (DFG PA 664/4-1) and the European Union under the SYNTHESYS-project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palm, H.W., Waeschenbach, A. & Littlewood, D.T.J. Genetic diversity in the trypanorhynch cestode Tentacularia coryphaenae Bosc, 1797: evidence for a cosmopolitan distribution and low host specificity in the teleost intermediate host. Parasitol Res 101, 153–159 (2007). https://doi.org/10.1007/s00436-006-0435-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-006-0435-1