Abstract
Hepatitis E is an emerging zoonotic infection of increasing public health threat for the UK, especially for immunosuppressed individuals. A human recombinant vaccine has been licensed only in China and is not clear whether it protects against hepatitis E virus (HEV) genotype 3, the most prevalent in Europe. The aim of this study was to use phage display technology as a tool to identify peptides that mimic epitopes of HEV capsid (mimotopes). We identified putative linear and conformational mimotopes using sera from Scottish blood donors that have the immunological imprint of past HEV infection. Four mimotopes did not have homology with the primary sequence of HEV ORF2 capsid but competed effectively with a commercial HEV antigen for binding to anti-HEV reference serum. When the reactivity profile of each mimotope was compared with Wantai HEV-IgG ELISA, the most sensitive HEV immunoassay, mimotopes showed 95.2–100% sensitivity while the specificity ranged from 81.5 to 95.8%. PepSurf algorithm was used to map affinity-selected peptides onto the ORF2 crystal structure of HEV genotype 3, which predicted that these four mimototopes are clustered in the P domain of ORF2 capsid, near conformational epitopes of anti-HEV neutralising monoclonal antibodies. These HEV mimotopes may have potential applications in the design of structural vaccines and the development of new diagnostic tests.
Similar content being viewed by others
Introduction
Historically, hepatitis E virus (HEV) infection in developing countries tended to be associated with acute outbreaks, caused by contamination of drinking water with sewage. Furthermore, sporadic infections were underdiagnosed due to the lack of affordable diagnostic tests with good performance and the co-circulation with HAV, another waterborne virus of similar clinical presentation [1, 2]. Hepatitis E acute outbreaks of countries without a clean water supply system were caused mainly by HEV genotypes 1 and 2 and occurred only in humans [3]. More recently, zoonotic infection caused by genotypes 3 and 4 has been described in industrialised countries as autochthonous infections rather than travel-associated [4]. In Scotland, the number of reported cases of hepatitis E has increased 14 times from 2011 to 2015 [5] and transmission via blood transfusion has become a concern, specially for immunosuppressed patients [6]. The precise sources of infection for the autochthonous cases are currently unknown, but increased anti-HEV IgG seroprevalence has been described among people exposed to pigs [7, 8], a group of patients with psychiatric disorders [9], homosexual men [10], amongst other food chain risk factors [6, 11]. In addition, transfusion-transmitted hepatitis E has been reported in the UK with an overall transmission rate of 42% [12].
HEV usually causes a self-limiting infection in immunocompetent individuals but HEV genotype 3 can cause chronic hepatitis in immunocompromised such as transplant recipients [13]. Increasing number of reports points also at potential extrahepatic complications such as neurological symptoms [14]. The mortality rate is approximately 2%. However, high mortality rates from HEV genotype 1 has been reported in pregnant women in India [3]. In UK, the clinical cases are prevalent in older males, caused mostly by genotype 3 [12, 15–17].
HEV is a small non-enveloped virus with a 7.2 kb RNA genome that contains three open reading frames [18]. ORF1 encodes for non-structural proteins and ORF3 for a phosphoprotein that appears to be involved in virion morphogenesis and HEV pathogenicity [19]. The only structural protein, the capsid is encoded by ORF2. Based on the crystal structure of virus like particles (VLP), the HEV capsid is considered to be formed by three domains: an S domain (amino acid (aa) 112–313), M domain (aa 314–454) and P domain (aa 455–606) [20]. The P domain forms a homodimer that protrudes from the basal shell of the virus capsid to form a spike; which is responsible for cell attachment and harbours the major neutralising B cell epitopes that are highly dependant on its 3D conformation [19].
There are a large number of approaches to vaccine development and generation of diagnostic tools [21–23]. In the case of HEV, most of the commercial and in-house serological assays are based on selected regions of immobilised ORF2 capsid protein, and some include ORF3 protein [24, 25]. The strategies for HEV vaccine development has been concentrated in the expression of several truncated recombinant ORF2 capsid proteins as immunogens. The most successful candidate was a recombinant protein of 239 amino acids (aa 368–606) from HEV genotype 1, expressed in Escherichia coli as VLP, which is has been licensed only in China under the trade name of Hecolin [19, 26]. This vaccine had an efficacy of 100% in participants receiving all three doses. The long term efficacy of Hecolin for up to 4.5 years was 86.8% [27]. However, it is unknown whether this vaccine provide protection against genotype 3 virus strains and patients at risk for chronic HEV [11]. In addition, antiviral treatment in immunocompromised patients might induce mutations in ORF2 gene, that could lead to the emergence of HEV vaccine escape mutants, as it has been reported for HBV [28–30].
Several studies has shown that phage display can be an important tool for epitope mapping, the development of vaccines, therapeutic drugs, diagnostic reagents, as well as in proteomic studies [31–35]. This technique is very useful to study viruses which do not replicate efficiently in tissue culture like HEV [36]. Strategies to identify peptides that mimic disease specific epitopes from phage-displayed random peptide libraries using human sera has been previously reported for several viruses [31, 32, 37]. Such phage-displayed peptides (mimotopes) do not necessarily have sequence homology with the antigen, but have sufficient conformational homology to induce high affinity antibodies that bind to both the mimotope and the natural antigen. Here, we described the identification of putative linear and conformational epitopes of HEV capsid using a combinatorial phage display library and sera from blood donors that have inapparent HEV infection.
Materials and methods
Sample collection
This was a retrospective study, involving serum samples from our research anonymous archive of consented Scottish blood donors [17]. These samples were previously tested by anti-HEV IgM and IgG commercial ELISA Kits (Beijing Wantai Biological Pharmacy Enterprise Ltd) and by “in house” HEV PCR [17]. Serum panel consisted of 25 anti-HEV IgG positive samples with anti-HEV levels above 1 WHO units per mL (IU/mL) and 29 anti-HEV IgG negative samples. All samples were negative for anti-HEV IgM and HEV RNA.
Affinity selection
The biopanning procedure has been described elsewhere [32] with some modifications. Briefly, each biopanning round consisted of a double selection strategy [37] of a dodecapeptide (Ph.D.-12) phage display library (New England BioLabs). The positive selection was carried out with protein G magnetic micro beads (Miltenyi Biotec) labelled with human IgG anti-HEV positive serum. The eluted bound phage was negatively selected with beads labelled with human IgG anti-HEV negative serum. Unbound phage from negative selection was amplified in E. coli ER2738 by a high-throughput method [38] and subjected to one more round of biopanning with different positive and negative sera. Input and ouput phages were titrated, and single phage clones were picked and tested by ELISA.
Sandwich phage ELISA
Multi-well plates (Nunc Maxisorp F8, Life Technologies) were coated overnight (4 °C) with anti-HEV positive serum at 1:500 dilution in carbonate-bicarbonate buffer (Sigma-Aldrich) and blocked for 1 h at room temperature with 3% BSA in PBS/0.05% Tween 20 (PBS-T). Plates were washed 3 times with PBS-T and 50 µL of high throughput phage clones (107–108 pfu/mL) were added in duplicate. As a negative control, M13KE wild-type (wt) phage without peptide insert was included at the same concentration. Plates were incubated for 2 h at 37 °C, washed four times and bound phages were detected using 1:1 000 dilution of anti-M13 horseradish peroxidase monoclonal conjugate (GE Healthcare) in 1% BSA in PBS-T. Ultra TMB ELISA substrate solution (Thermo Scientific) was added after 5 washes with PBS-T. The reaction was stopped after 15 min with 1 N H2SO4 and read at 450 nm against the blank (LB medium with tetracycline at 10 µg/mL). Optical density (OD) signals at least 3 times higher than signals detected with the wild-type phage were considered positive, and the reactivity of each phagotope was expressed as a colour coded heat map.
To investigate non-specific binding to plastic, Nunc Maxisorp F8 wells were coated with carbonate-bicarbonate buffer without antibody. Some strips were blocked either with 3% BSA in PBS-T or PBS-T alone. The rest of the sandwich phage ELISA procedure was carried out as described above.
Indirect sandwich phage ELISA
This ELISA was used to test mimotope reactivity against a panel of human sera, as described elsewhere [32] with the exception that cut-off results were calculated by ROC curve analysis, which generated a sensitivity/specificity report against Wantai HEV-IgG ELISA results.
Competitive inhibition assay of HEV mimotopes
Briefly, 20 μL of each phagotope at 1013 pfu/mL and 20 μL of anti-HEV serum at 2 IU/mL were incubated in 200 μL of ELISA’s specimen diluent overnight at 37 °C. A calibration curve was obtained by four parameter logistic fit of two fold serial dilutions of WHO 95/584 anti-HEV reference serum (Ascent software for Multiskan microplate reader). The anti-HEV antibody concentration was measured before and after phagotope adsorption using Wantai HEV-IgG ELISA, which has a lower limit of detection for HEV IgG of 0.2 IU/mL [39, 40]. The formula used for calculating the percentage of anti-HEV antibody inhibition by the phage clones is described elsewhere [32]. Samples were evaluated in duplicate and the experiment was performed twice.
Sequencing analysis
Double stranded replicative form DNA of phage clones was purified as described [32] and sequenced by Eurofins Genomics GmbH using NEB’s -96 gIII sequencing primer (5´-CCC TCA TAG TTA GCG TAA CG-3´) and -28 gIII sequencing primer (5´-GTA TGG GAT TTT GCT AAA CAA C-3´). Phage-displayed peptide sequences were deducted and aligned using Geneious Pro 5.6.2 software [41]. SAROTUP and MimoDB databases [42, 43] were searched to predict the presence of target-unrelated peptides (TUPs).
Mimotope 3D mapping
PepSurf algorithm [44] of the Pepitope server was used to map affinity-selected peptides onto the ORF2 crystal structure of HEV genotype 3 (PDB no. 2ZTN). PepSurf aligns each peptide to a graph which represents the surface of the input 3D structure. Amino acid similarities are scored using Blosum62. Each aligned peptide corresponds to a path of residues on the 3D structure that exhibits a high similarity to the input peptide.
Statistics
MedCalc statistical software (version 13.0) was used for ROC curve analysis and Mann–Whitney U test.
Results
Sera with high anti-HEV levels (378 and 101 IU/mL, respectively) were used for the first and second rounds of biopanning. The yield after the first round was within the expected range for biopaning rounds (10−4–10−8). Since the enrichment after the second round was low (Suppl. Table 1), we decided to perform two selection rounds and test a large number of phage clones [31].
In total 368 phage clones were tested by sandwich phage ELISA (Table 1). 10.3% of the clones displaying peptide epitopes (phagotopes) were reactive at least with one of the two anti-HEV positive sera used in the initial screening. Only the phagotopes that were reactive with both S51 and S56 anti-HEV positive sera and have a clone/wt ratio above 5 with at least one serum were chosen for further mimotope screening. These phagotopes were renamed with the abbreviation M for mimotope plus consecutive numbers for easier identification. The colour coded heat map shows that phagotopes with a large range of reactivity from low to very strong were chosen for further testing with a panel of sera.
TUPs scan in SAROTUP found only partial homology within the three amino acid sequence (SSL) of dodecapeptide M13 with the peptide FARLVSSIRY, which compete for the protein A binding site [45]. No hits were found in the MimoDB database for the phagotopes described in Table 1. Based on the prediction of these phage display databases is unlikely that the phagotopes were TUPs.
Clones were then tested for plastic and BSA binding (Suppl. Figure 1). Phagotopes M1, M8, M9, M11 and M12 with a mean absorbance higher than 0.1 were discarded from the study due to the high ELISA background signal. In general, the binding to unblocked plastic (PBST) was higher than in wells coated with BSA.
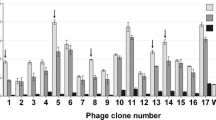
The reaction of the phagotopes with anti-HEV antibody is shown in Fig. 1 as the percent inhibition of binding of WHO anti-HEV reference serum to HEV ORF2 antigen compared with the unabsorbed sera. There was no significant difference in the mean of inhibition % between M4, M5, M6 and wt phage. Mimotopes M2, M3, M7, M10, M13 and M14 significantly inhibited anti-HEV antibody compared with wt phage (Mann–Whitney U test, p = 0.021). The highest inhibition percent values were achieved by M7 and M10. Thus, these six mimotopes seem to compete effectively with HEV ORF2 antigen for binding to specific antibodies.
Mimotopes M2, M3, M7, M10, M13 and M14 compete with HEV ORF2 antigen for binding to specific antibodies. Mimotopes were tested by a competitive inhibition assay. Anti-HEV antibody concentration was measured before and after phagotope absorption using Wantai HEV-IgG ELISA. A calibration curve was obtained by four parameter logistic fit (Suppl. Figure 2c). An asterisk (*) marks the mimotopes that significantly inhibited anti-HEV antibody levels compared with wt phage inhibition (Mann–Whitney U test, p = 0.021)
Sera from 48 blood donors were then used to test the mimotopes by indirect sandwich phage ELISA. None of the sera reacted to wt phage since their absorbance at 450 nm was below background levels (Suppl. Figure 2a). The median of anti-HEV levels was 8.6 IU/mL, the lowest value was 1.2 IU/mL and the highest was 88.1 IU/mL (Suppl. Figure 2b). ROC curve analysis was used to compare mimotope detection by this “in house” ELISA with Wantai HEV-IgG ELISA (Fig. 2). Most of the mimotopes had recommended cut-off (CO) less than 0.1, except M2. To avoid noise signal, we normally set up the CO values in our “in house” ELISAs bellow 0.1. Therefore, we could assume that a more realistic specificity for M2 is 91.7% if the CO is set up at 0.1. The majority of the mimotopes showed 100% sensitivity, except M14 (95.2%). However, the specificity ranged from 81.5 to 95.8%.
Interactive dot diagrams (ROC curve analysis) showing phagotopes ELISA reactivity to a panel of 27 anti-HEV negative sera (0) and 21 anti-HEV positive sera (1). MedCalc recommended cut-offs are indicated as a solid blue line. Sensitivity and specificity in comparison to Wantai HEV-IgG ELISA is shown for each phagotope under the cut-off value (colour figure online)
To map linear epitopes, phage-displayed peptide sequences were aligned against the consensus sequence of 21 HEV strains (Suppl. Table 2), which are representative of the four HEV genotypes. Mimotope M3 had homology with the 219PTSVD223 ORF2 region (Fig. 3a). Amino acids 200–240 are 100% conserved amongst these human and swine HEV strains. This suggests that M3 could mimic a linear epitope of HEV capsid. However, M14 showed poor homology within the region 465–475, which is also highly conserved. W472R is the only natural substitution observed in this region. No homology in the primary sequence was found with the rest of the mimotopes.
Identification of linear and conformational mimotopes of the HEV capsid. a Deduced amino acid sequences of M3 and M14 mimotopes and alignment with the capsid of human and swine HEV. 21 HEV strains that are representative of genotypes 1, 2, 3 and 4 were used to obtain a consensus ORF2 sequence. b Mimotope sequences (M2, M7, M10 and M13) were mapped onto the ORF2 crystal structure of HEV genotype 3 (PDB no. 2ZTN) and visualised in FirstGlance in Jmol. The peptides alignment region is shown in red (PepSurf algorithm). Key amino acids of epitopes recognised by neutralising MAbs 8G12, 1323, and 8C11 are shown in gray with yellow halos. The 3D structure of ORF2 monomers that form the capsid was magnified, showing mostly the P domain that protrudes from the basal shell of the virus to form a spike. The four images are produced by rotation of the 3D protein structure
PepSurf [44] was used to map the mimotopes onto the ORF2 crystal structure of HEV genotype 3 (PDB no. 2ZTN). This algorithm predicted that mimototopes M2, M7, M10 and M13 are clustered in the P domain of the ORF2 capsid (Fig. 3b). M2 maps very close to Glu549, Lys554 and Gly591 conserved residues that are essential for neutralisation of monoclonal antibody (MAb) 8G12 [46]. M7 is closely located to residues Ser487, Ser488, Thr489, Pro491, Asn562 and Thr564 in the peripheral region of the apical surface recognised by MAb1323 [20]. Notice that Ser11 of mimotope M7 seems to mimic Thr564, involved in binding to host cell [20]. M10 and M13 are located in the groove region, near residues Glu479, Tyr485, Asp496, Arg512, Lys534, His577 and Arg578 recognised by MAb 8C11. Mimotope M10 shares His577, which is also involved in binding to host cell [46–48].
Discussion
The aim of this study was to investigate peptides that mimic epitopes of HEV capsid (mimotopes) for their vaccine and diagnostic potential. NEB’s Ph.D.-12 phage display library (PDL) derived from M13mp19 vector was selected as a source of epitopes. This library consists of 1.9 × 109 unique phage clones displaying randomised 12-mer peptides fused to pIII protein via the flexible linker GGGS without affecting significantly the phage infectivity. These 12-mer peptides are long enough to fold into short structural elements, which may be useful when panning against targets that require structured ligands like the HEV neutralisation site. We have chosen a double selection strategy for each round of biopanning that was designed to differentiate between uninfected subjects with naive IgG and individuals with anti-HEV IgG levels above 1 IU/mL, who might have suffered asymptomatic HEV infection and could be more protected than those with marginal antibody levels [49].
We decided to perform only two rounds of biopanning (Suppl. Table 1), since we have observed no enrichment after a second round of affinity selection in the past while using human sera as a target for identifying HAV mimotopes [32].
A common feature of plastic binding peptides is a high abundance of aromatic amino acid residues (Phe, Tyr and Trp) which are crucial for hydrophobic interactions [50]. Peptides M1, M8, M11 and M12 adsorbed non-specifically to polystyrene surface, even after blocking with 3% BSA in PBS-T (Suppl. Figure 1). Two Trp residues separated by one or more random amino acids frequently occur in plastic binders [50] like in M8 and M11, where the presence of other hydrophobic residues might contribute to a stronger background signal. Peptides that do not have aromatic residues (M12) but are abundant in Ser may also bind to bovine serum albumin [50].
M13 did not bind plastic or BSA in our experimental conditions (Suppl. Figure 1) despite it was a suspected binder to immunoglobulin Fc (matched pattern SS[IL]) in SAROTUP. After absorption with M13, around 45% of anti-HEV antibody levels were inhibited; and this competitive inhibition was significant compared with wt phage (Fig. 1). Three false positive results were obtained after testing M13 mimotope by indirect sandwich phage ELISA, with a specificity of 87.5% (Fig. 2). This confirms previous warnings about possible false negative and false positive cleaning results with SAROTUP [50].
Initial sandwich phage ELISA screening results (Table 1) were confirmed by a competitive inhibition assay that uses recombinant ORF2 protein as the antigen to compete with phagotopes for anti-HEV reference serum binding [24]. Six mimotopes efficiently competed with HEV for binding to specific antibodies (Fig. 1), which supports algorithms that predicted that four mimotopes mimic conformational epitopes of the P region of HEV capsid (Fig. 3b). In addition, the reactivity profile of each mimotope in an indirect sandwich phage ELISA was compared to the Wantai HEV-IgG ELISA [6]. Most mimotopes showed 100% sensitivity for the detection of anti-HEV positive samples, except M14 (95.2%). However, the specificity ranged from 81.5 to 95.8%.
Although the biopanning procedure consisted of a double selection strategy with anti-HEV positive and negative sera; the selector is a large collection of polyclonal antibodies that are poly specific and could enrich phagotopes which are not disease specific [31]. Results shown in Table 1 are after initial screening by sandwich phage ELISA, using only two anti-HEV positive sera (S51 and S56). Thus, the very strong reactivity shown for putative mimotopes M1 and M12 seems to be due to plastic binding (Supplemental Fig. 1). In the case of M5, no plastic binding was detected, and it has not been described as a target-unrelated peptide (TUP) by SAROTUP and MimoDB databases. However, M5 very strong reactivity by sandwich phage ELISA (Table 1) also seems to be a false positive result, typical of a TUP, which might be attributed to binding to the Fc region of anti-HEV antibodies on the solid phase, amongst other factors [42, 43]. The competitive inhibition assay confirmed that M5 peptide is unable to inhibit the binding of WHO international anti-HEV reference serum (NIBSC 95/584) to recombinant HEV ORF2 protein in Wantai HEV-IgG ELISA (Fig. 1) [24, 51]. This indirect ELISA is the commercial assay with the highest analytical sensitivity (0.2 IU/mL) and specificity of 97.8–99.6% (depending on “in house” specificity panels) [6, 40, 52].
We studied a sample of blood donors that were probably asymptomatic infected by HEV in the past since all serum samples were negative for anti-HEV IgM and HEV RNA. The median of anti-HEV levels was 8.6 IU/mL, which is in accordance with a geometric mean concentration of 2.06 ± 6.30 IU/mL found in asymptomatic infected individuals in a large vaccine trial [49]. This contrasts with the stronger response attributed to primary symptomatic infection which has a mean around 80.9 IU/mL [53]. Specificity is difficult to assess in situations other than acute hepatitis E because there is no gold standard for checking the specificity of the current anti-HEV ELISA kits [39, 54, 55]. Thus, we could not confirm if the false positive results obtained are in fact true positive since is well known that screening assays with very high sensitivity are prone to false positive.
Most of reported HEV neutralising epitopes are conformational with the exception of amino acids 423–438 and 578–607, which are recognised by MAbs 12A10 and HEV#4/HEV#31, respectively [46]. Linear epitope (aa 423–438) is involved in virus attachment to the host cell but is not immunogenic in natural HEV infections [46, 56]. The second putative linear epitope was identified by a chimpanzee antibody phage library. Both HEV#4 and HEV#31 Fabs neutralised the SAR-55 strain of HEV in vivo [57]. However, the authors concluded that the epitopes at the ORF2 C-terminus are conformational based in their failure to develop an ELISA based on the aa589–607 peptide and the inability of this peptide to compete for binding in radio immunoprecipitation assay [58]. Sequencing analysis predicts that the PSTLD sequence of mimotope M3 (Fig. 3a) might mimic a putative linear epitope of HEV since it was recognised by all anti-HEV positive sera. However, this data awaits conformation since M3 was the mimotope with the lowest specificity (81.5%) and the immune response of most of the sera was weak (Fig. 2). Additional experiments are required to confirm whether the 219PTSVD223 ORF2 region is immunogenic in natural HEV infections.
PepSurf predicted that mimototopes M2, M7, M10 and M13 are clustered in the P domain of the ORF2 capsid, which is responsible for cell-host interactions, and harbours the major neutralisation B-cell epitopes [46]. Residues Tyr537, Leu545, Phe547 mapped by M2 form part of the T-cell epitope (aa 537–551), previously identified with overlapping peptides [19]. Neutralising MAb 8C11 was previously used to select HPTLLRI peptide from NEB’s Ph.D.-7 PDL [59]. Interestingly, M10 and M13 mimotopes are located near residues recognised by this neutralising antibody and in particularly M10 shares His577 with 8C11 that is involved in cell attachment [19, 46, 47]. Also mimotope M7 maps near residues of MAb1323, which is suggested to directly inhibit the interaction between HEV VLP and cellular receptors, through binding to the apical surface [20]. This is the first report of using the natural immune response of human sera to select phage displayed peptides that seem to mimic epitopes of HEV capsid (mimotopes) that may have future diagnostic and vaccine applications.
References
Rodrıguez-Lay L, Quintana A, Montalvo-Villalba MC et al (2008) Dual infection with hepatitis A and E viruses in outbreaks and in sporadic clinical cases: Cuba 1998–2003. J Med Virol 80:798–802. doi:10.1002/jmv.21147
Quintana A, Sanchez L, Larralde O, Anderson D (2005) Prevalence of antibodies to hepatitis E virus in residents of a district in Havana, Cuba. J Med Virol 76:69–70. doi:10.1002/jmv.20324
Park WJ, Park BJ, Ahn HS et al (2016) Hepatitis E virus as an emerging zoonotic pathogen. J Vet Sci 17:1–11. doi:10.4142/jvs.2016.17.1.1
Dreier J, Juhl D (2014) Autochthonous hepatitis e virus infections: a new transfusion-associated risk? Transfus Med hemotherapy 41:29–39. doi:10.1159/000357098
Health Protection Scotland (2016) Hepatitis E. HPS Wkly Rep 50:103–107
Petrik J, Lozano M, Seed CR et al (2016) Hepatitis E. Vox Sang 110:93–130. doi:10.1111/vox.12285
Teixeira J, Mesquita JR, Pereira SS et al (2017) Prevalence of hepatitis E virus antibodies in workers occupationally exposed to swine in Portugal. Med Microbiol Immunol 206:1–5. doi:10.1007/s00430-016-0484-8
Krumbholz A, Joel S, Dremsek P et al (2014) Seroprevalence of hepatitis E virus (HEV) in humans living in high pig density areas of Germany. Med Microbiol Immunol 203:273–282. doi:10.1007/s00430-014-0336-3
Reinheimer C, Allwinn R, Berger A (2012) Hepatitis E: are psychiatric patients on special risk? Med Microbiol Immunol 201:171–175. doi:10.1007/s00430-011-0218-x
Payne BA, Medhi M, Ijaz S et al (2013) Hepatitis E virus seroprevalence among men who have sex with men, United Kingdom. Emerg Infect Dis 19:333–335. doi:10.3201/eid1902.121174
Kmush BL, Nelson KE, Labrique AB (2015) Risk factors for hepatitis E virus infection and disease. Expert Rev Anti Infect Ther 13:41–53. doi:10.1586/14787210.2015.981158
Hewitt PE, Ijaz S, Brailsford SR et al (2014) Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet 6736:1–8. doi:10.1016/S0140-6736(14)61034-5
Khuroo MS, Khuroo MS, Khuroo NS (2016) Hepatitis E: discovery, global impact, control and cure. World J Gastroenterol 22:7030–7045. doi:10.3748/wjg.v22.i31.7030
Kamar N, Bendall RP, Peron JM et al (2011) Hepatitis E virus and neurologic disorders. Emerg Infect Dis 17:173–179. doi:10.3201/eid1702.100856
Ijaz S, Said B, Boxall E et al (2014) Indigenous hepatitis E in England and wales from 2003 to 2012: evidence of an emerging novel phylotype of viruses. J Infect Dis 209:1212–1218. doi:10.1093/infdis/jit652
Kokki I, Smith D, Simmonds P et al (2016) Hepatitis E virus is the leading cause of acute viral hepatitis in Lothian, Scotland. New Microbes New Infect 10:6–12. doi:10.1016/j.nmni.2015.12.001
Cleland A, Smith L, Crossan C et al (2013) Hepatitis E virus in Scottish blood donors. Vox Sang 105:283–289. doi:10.1111/vox.12056
Pérez-Gracia MT, Suay B, Mateos-Lindemann ML (2014) Hepatitis E: an emerging disease. Infect Genet Evol 22:40–59. doi:10.1016/j.meegid.2014.01.002
Li S, Zhang J, Xia N (2015) Lessons from hepatitis E vaccine design. Curr Opin Virol 11:130–136. doi:10.1016/j.coviro.2015.04.003
Yamashita T, Mori Y (2009) Biological and immunological characteristics of hepatitis E virus-like particles based on the crystal structure. PNAS 106:12986–12991. doi:10.1073/pnas.0903699106
Doerr HW, Berger A (2014) Vaccination against infectious diseases: what is promising? Med Microbiol Immunol 203:365–371. doi:10.1007/s00430-014-0346-1
Delany I, Rappuoli R, De Gregorio E (2014) Vaccines for the 21st century. EMBO Mol Med 6:708–720. doi:10.1002/emmm.201403876
Sarkander J, Hojyo S, Tokoyoda K (2016) Vaccination to gain humoral immune memory. Clin Transl Immunol 5:1–6. doi:10.1038/cti.2016.81
Zhao Q, Zhang J, Wu T et al (2013) Antigenic determinants of hepatitis E virus and vaccine-induced immunogenicity and efficacy. J Gastroenterol 48:159–168. doi:10.1007/s00535-012-0701-1
Dremsek P, Wenzel JJ, Johne R et al (2012) Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Med Microbiol Immunol 201:189–200. doi:10.1007/s00430-011-0221-2
Li SW, Zhao Q, Wu T et al (2015) The development of a recombinant hepatitis E vaccine HEV 239. Hum Vaccines Immunother 11:908–914. doi:10.1080/21645515.2015.1008870
Zhang J, Zhang X-F, Huang S-J et al (2015) Long-term efficacy of a hepatitis E vaccine. N Engl J Med 372:914–922. doi:10.1056/NEJMoa1406011
van Tong H, Hoan NX, Wang B et al (2016) Hepatitis E virus mutations: functional and clinical relevance. EBioMedicine 11:31–42. doi:10.1016/j.ebiom.2016.07.039
Larralde O, Dow B, Jarvis L et al (2013) Hepatitis B escape mutants in Scottish blood donors. Med Microbiol Immunol 202:207–214. doi:10.1007/s00430-012-0283-9
Todt D, Gisa A, Radonic A et al (2016) In vivo evidence for ribavirin-induced mutagenesis of the hepatitis E virus genome. Gut. doi:10.1136/gutjnl-2015-311000
Folgori A, Tafi R, Meola A et al (1994) A general strategy to identify mimotopes of pathological antigens using only random peptide libraries and human sera. EMBO J 13:2236–2243
Larralde OG, Martinez R, Camacho F et al (2007) Identification of hepatitis A virus mimotopes by phage display, antigenicity and immunogenicity. J Virol Methods 140:49–58. doi:10.1016/j.jviromet.2006.10.015
Sundell GN, Ivarsson Y (2014) Interaction analysis through proteomic phage display. Biomed Res Int 2014:176172. doi:10.1155/2014/176172
Bakhshinejad B, Sadeghizadeh M (2014) Bacteriophages and their applications in the diagnosis and treatment of hepatitis B virus infection. World J Gastroenterol 20:11671–11683. doi:10.3748/wjg.v20.i33.11671
Tan WS, Ho KL (2014) Phage display creates innovative applications to combat hepatitis B virus. World J Gastroenterol 20:11650–11670. doi:10.3748/wjg.v20.i33.11650
Okamoto H (2013) Culture systems for hepatitis E virus. J Gastroenterol 48:147–158. doi:10.1007/s00535-012-0682-0
Bachler BC, Humbert M, Palikuqi B et al (2013) Novel biopanning strategy to identify epitopes associated with vaccine protection. J Virol 87:4403–4416. doi:10.1128/JVI.02888-12
Yu X, Owens GP, Gilden DH (2006) Rapid and efficient identification of epitopes/mimotopes from random peptide libraries. J Immunol Methods 316:67–74. doi:10.1016/j.jim.2006.08.006
Bendall R, Ellis V, Ijaz S (2010) A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol 805:799–805. doi:10.1002/jmv
Vollmer T, Diekmann J, Eberhardt M et al (2016) Monitoring of anti-hepatitis E virus antibody seroconversion in asymptomatically infected blood donors: systematic comparison of nine commercial anti-HEV IgM and IgG assays. Viruses 8:232. doi:10.3390/v8080232
Kearse M, Moir R, Wilson A et al (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649
Huang J, Ru B, Li S et al (2010) SAROTUP: scanner and reporter of target-unrelated peptides. J Biomed Biotechnol 2010:101932. doi:10.1155/2010/101932
Huang J, Ru B, Zhu P et al (2012) MimoDB 2.0: a mimotope database and beyond. Nucleic Acids Res 40:D271–D277. doi:10.1093/nar/gkr922
Mayrose I, Shlomi T, Rubinstein ND et al (2007) Epitope mapping using combinatorial phage-display libraries: a graph-based algorithm. Nucleic Acids Res 35:69–78. doi:10.1093/nar/gkl975
Krook M, Mosbach K, Ramström O (1998) Novel peptides binding to the Fc-portion of immunoglobulins obtained from a combinatorial phage display peptide library. J Immunol Methods 221:151–157. doi:10.1016/S0022-1759(98)00177-X
Gu Y, Tang X, Zhang X et al (2015) Structural basis for the neutralization of hepatitis E virus by a cross-genotype antibody. Cell Res 25:604–620. doi:10.1038/cr.2015.34
Tang X, Yang C, Gu Y (2011) Structural basis for the neutralization and genotype specificity of hepatitis E virus. PNAS 108:10266–10271. doi:10.1073/pnas.1101309108
Zhang J, Gu Y, Ge SX et al (2005) Analysis of hepatitis E virus neutralization sites using monoclonal antibodies directed against a virus capsid protein. Vaccine 23:2881–2892. doi:10.1016/j.vaccine.2004.11.065
Zhang J, Zhang X-F, Zhou C et al (2014) Protection against hepatitis E virus infection by naturally acquired and vaccine-induced immunity. Clin Microbiol Infect 20:O397–O405. doi:10.1111/1469-0691.12419
Vodnik M, Zager U, Strukelj B, Lunder M (2011) Phage display: selecting straws instead of a needle from a haystack. Molecules 16:790–817. doi:10.3390/molecules16010790
Ferguson M, Walker D, Mast E, Fields H (2002) Report of a collaborative study to assess the suitability of a reference reagent for antibodies to hepatitis E virus. Biologicals 30:43–48. doi:10.1006/biol.2001.0315
Abravanel F, Chapuy-Regaud S, Lhomme S et al (2013) Performance of anti-HEV assays for diagnosing acute hepatitis E in immunocompromised patients. J Clin Virol 58:624–628. doi:10.1016/j.jcv.2013.10.003
Zhang J, Li S-W, Wu T et al (2012) Hepatitis E virus: neutralizing sites, diagnosis, and protective immunity. Rev Med Virol 22:339–349. doi:10.1002/rmv.1719
Ahmed A, Ali IA, Ghazal H et al (2015) Mystery of hepatitis e virus: recent advances in its diagnosis and management. Int J Hepatol 2015:872431. doi:10.1155/2015/872431
Park HK, Jeong S-H, Kim J-W et al (2012) Seroprevalence of anti-hepatitis E virus (HEV) in a Korean population: comparison of two commercial anti-HEV assays. BMC Infect Dis 12:142. doi:10.1186/1471-2334-12-142
He S, Miao J, Zheng Z et al (2008) Putative receptor-binding sites of hepatitis E virus. J Gen Virol 89:245–249. doi:10.1099/vir.0.83308-0
Schofield D, Glamann J (2000) Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J Virol 74:5548–5555. doi:10.1128/JVI.74.12.5548-5555.2000
Schofield DJ, Purcell RH, Nguyen HT, Emerson SU (2003) Monoclonal antibodies that neutralize HEV recognize an antigenic site at the carboxyterminus of an ORF2 protein vaccine. Vaccine 22:257–267. doi:10.1016/j.vaccine.2003.07.008
Gu Y, Zhang J, Wang YB et al (2004) Selection of a peptide mimicking neutralization epitope of hepatitis E virus with phage peptide display technology. World J Gastroenterol 10:1583–1588. doi:10.3748/wjg.v10.i11.1583
Acknowledgements
This work was funded by NHS National Services Scotland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All blood donors were voluntary and non-remunerated. Anonymous data was used in the study protocol in order to protect confidentiality.
Electronic supplementary material
Below is the link to the electronic supplementary material.
430_2017_507_MOESM1_ESM.tif
Suppl. Figure 1 Elimination of plastic binding clones. Sandwich phage ELISA modification: wells were coated with carbonate-bicarbonate buffer without anti-HEV + serum and blocked with either 3% BSA in PBS-T (grey error bars) or PBS-T (black error bars). Error bars show mean plus the standard deviation of two replicate experiments (TIFF 683 kb)
430_2017_507_MOESM2_ESM.tif
Suppl. Figure 2 Data supporting indirect sandwich phage ELISA and ROC curve analysis, used to test mimotope reactivity against a panel of human sera. (a) Frequency distribution of reactivity against wild-type phage (M13KE) for the sera panel used for mimotope testing (n = 48). None of the sera reacted to wt phage in the indirect sandwich phage ELISA since their absorbance at 450 nm was below background levels (< 0.1). (b) Boxplot showing non-normal distribution of the anti-HEV antibody levels (IU/mL), measured using Wantai HEV-IgG ELISA. The anti-HEV positive sera (n = 21) used for testing mimotope reactivity had a broad range of anti-HEV titres (1.2-88.1 IU/mL) with a median of 8.6 IU/mL. (c) Example of calibration curve to calculate anti-HEV antibody levels using four parameter logistic fit of two fold serial dilutions of WHO anti-HEV reference serum in Ascent software. Anti-HEV concentration vs absorbance at 450 nm is depicted in logarithmic scale. The correlation coefficient of replicate experiments was higher than 0.97 (TIFF 1251 kb)
Rights and permissions
About this article
Cite this article
Larralde, O., Petrik, J. Phage-displayed peptides that mimic epitopes of hepatitis E virus capsid. Med Microbiol Immunol 206, 301–309 (2017). https://doi.org/10.1007/s00430-017-0507-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-017-0507-0