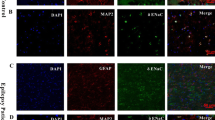
Abstract
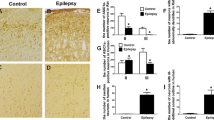
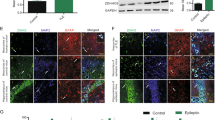
Synaptic reorganization in the epileptic hippocampus involves altered excitatory and inhibitory transmission besides the rearrangement of dendritic spines, resulting in altered excitability, ion homeostasis, and cell swelling. The potassium-chloride cotransporter-2 (KCC2) is the main chloride extruder in neurons and hence will play a prominent role in determining the polarity of GABAA receptor-mediated chloride currents. In addition, KCC2 also interacts with the actin cytoskeleton which is critical for dendritic spine morphogenesis, and for the maintenance of glutamatergic synapses and cell volume. Using immunocytochemistry, we examined the cellular and subcellular levels of KCC2 in surgically removed hippocampi of temporal lobe epilepsy (TLE) patients and compared them to control human tissue. We also studied the distribution of KCC2 in a pilocarpine mouse model of epilepsy. An overall increase in KCC2-expression was found in epilepsy and confirmed by Western blots. The cellular and subcellular distributions in control mouse and human samples were largely similar; moreover, changes affecting KCC2-expression were also alike in chronic epileptic human and mouse hippocampi. At the subcellular level, we determined the neuronal elements exhibiting enhanced KCC2 expression. In epileptic tissue, staining became more intense in the immunopositive elements detected in control tissue, and profiles with subthreshold expression of KCC2 in control samples became labelled. Positive interneuron somata and dendrites were more numerous in epileptic hippocampi, despite severe interneuron loss. Whether the elevation of KCC2-expression is ultimately a pro- or anticonvulsive change, or both—behaving differently during ictal and interictal states in a context-dependent manner—remains to be established.






Similar content being viewed by others
Abbreviations
- CA:
-
Cornu Ammonis
- DAB:
-
3,3‘diamino-benzidine 4 HCl
- DG:
-
Dentate gyrus
- EEG:
-
Electroencephalogram
- GABA:
-
Gamma-aminobutyric acid
- GluR1:
-
Glutamate receptor 1
- KCC2:
-
Potassium-chloride cotransporter-2
- PET:
-
Positron emission tomography
- Pilo:
-
Pilocarpine
- SE:
-
Status epilepticus
- Str:
-
Stratum
- SPECT:
-
Single-photon emission computerized tomography
- TLE:
-
Temporal lobe epilepsy
References
Aronica E et al (2007) Differential expression patterns of chloride transporters, Na+ -K+ -2Cl–cotransporter and K + -Cl–cotransporter, in epilepsy-associated malformations of cortical development. Neuroscience 145:185–196
Babb TL et al (1989) Glutamate decarboxylase-immunoreactive neurons are preserved in human epileptic hippocampus. J Neurosci 9:2562–2574
Baldi R, Varga C, Tamas G (2010) Differential distribution of KCC2 along the axo-somato-dendritic axis of hippocampal principal cells. Eur J Neurosci 32:1319–1325
Barmashenko G et al (2011) Positive shifts of the GABAA receptor reversal potential due to altered chloride homeostasis is widespread after status epilepticus. Epilepsia 52:1570–1578
Blackwood, W., Corsellis, J.A.N., 1976. Greenfield’s Neuropathology, Vol Edward Arnold, London
Bragin DE et al (2009) Development of epileptiform excitability in the deep entorhinal cortex after status epilepticus. Eur J Neurosci 30:611–624
Buckmaster PS, Dudek FE (1997) Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol 385:385–404
Cavalheiro EA, Santos NF, Priel MR (1996) The pilocarpine model of epilepsy in mice. Epilepsia 37:1015–1019
Cohen I et al (2002) On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science 298:1418–1421
Colder BW et al (1996) Decreased neuronal burst discharge near site of seizure onset in epileptic human temporal lobes. Epilepsia 37:113–121
Conti L et al (2011) Anomalous levels of Cl- transporters cause a decrease of GABAergic inhibition in human peritumoral epileptic cortex. Epilepsia 52:1635–1644
Corsellis JAN (1955) The incidence of Ammon’s horn sclerosis. Brain 80:193–208
Cossart R et al (2001) Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci 4:52–62
de Curtis M, Gnatkovsky V (2009) Reevaluating the mechanisms of focal ictogenesis: the role of low-voltage fast activity. Epilepsia 50:2514–2525
de Lanerolle NC et al (1988) Evidence for hippocampal interneuron loss in human temporal lobe epilepsy. Epilepsia 29:674
de Lanerolle NC et al (1989) Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res 495:387–395
de Lanerolle NC et al (2003) A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: evidence for distinctive patient subcategories. Epilepsia 44:677–687
Fiumelli H et al (2013) An ion transport-independent role for the cation-chloride cotransporter KCC2 in dendritic spinogenesis in vivo. Cereb Cortex 23:378–388
Freund TF, Buzsaki G (1996) Interneurons of the hippocampus. Hippocampus 6:347–470
Fujiwara-Tsukamoto Y et al (2003) Excitatory GABA input directly drives seizure-like rhythmic synchronization in mature hippocampal CA1 pyramidal cells. Neuroscience 119:265–275
Galanopoulou AS (2008) Dissociated gender-specific effects of recurrent seizures on GABA signaling in CA1 pyramidal neurons: role of GABA(A) receptors. J Neurosci 28:1557–1567
Gauvain G et al (2011) The neuronal K-Cl cotransporter KCC2 influences postsynaptic AMPA receptor content and lateral diffusion in dendritic spines. Proc Natl Acad Sci USA 108:15474–15479
Gnatkovsky V et al (2008) Fast activity at seizure onset is mediated by inhibitory circuits in the entorhinal cortex in vitro. Ann Neurol 64:674–686
Green, R.C., 1991. Neuropathology and behavior in epilepsy. In: Epilepsy and Behavior. Vol ed. Wiley pp 345–359
Gulyas AI et al (2001) The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur J Neurosci 13:2205–2217
Heinemann U et al (2000) Alterations of glial cell function in temporal lobe epilepsy. Epilepsia 41:S185–S189
Hoffmann EK, Dunham PB (1995) Membrane mechanisms and intracellular signalling in cell volume regulation. Int Rev Cytol 161:173–262
Houser CR (1991) GABA neurons in seizure disorders: a review of immunocytochemical studies. Neurochem Res 16:295–308
Huberfeld G et al (2007) Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci 27:9866–9873
Isokawa M (2000) Remodeling dendritic spines of dentate granule cells in temporal lobe epilepsy patients and the rat pilocarpine model. Epilepsia 41(Suppl 6):S14–S17
Jaenisch N, Witte OW, Frahm C (2010) Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. Stroke 41:e151–e159
Kahle KT et al (2013) Modulation of neuronal activity by phosphorylation of the K–Cl cotransporter KCC2. Trends Neurosci 36:726–737
Kaila K et al (1997) Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J Neurosci 17:7662–7672
Kaila K et al (2014a) Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci 15:637–654
Kaila K et al (2014b) GABA actions and ionic plasticity in epilepsy. Curr Opin Neurobiol 26:34–41
Karlocai MR et al (2011) Redistribution of CB1 Cannabinoid Receptors in the Acute and Chronic Phases of Pilocarpine-Induced Epilepsy. PLoS One 6:e27196
Lang F et al (1998) Functional significance of cell volume regulatory mechanisms. Physiol Rev 78:247–306
Laurberg S, Zimmer J (1981) Lesion-induced sprouting of hippocampal mossy fiber collaterals to the fascia dentata in developing and adult rats. J Comp Neurol 200:433–459
Li H et al (2007) KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron 56:1019–1033
Li X et al (2008) Long-term expressional changes of Na + -K + -Cl- co-transporter 1 (NKCC1) and K + -Cl- co-transporter 2 (KCC2) in CA1 region of hippocampus following lithium-pilocarpine induced status epilepticus (PISE). Brain Res 1221:141–146
Loscher W, Puskarjov M, Kaila K (2013) Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology 69:62–74
Lowenstein DH et al (1992) Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci 12:4846–4853
Magloczky Z (2010) Sprouting in human temporal lobe epilepsy: excitatory pathways and axons of interneurons. Epilepsy Res 89:52–59
Magloczky Z, Freund TF (1993) Selective neuronal death in the contralateral hippocampus following unilateral kainate injections into the CA3 subfield. Neuroscience 56:317–335
Magloczky Z, Freund TF (2005) Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci 28:334–340
Magloczky Z et al (1997) Loss of Calbindin-D28 K immunoreactivity from dentate granule cells in human temporal lobe epilepsy. Neuroscience 76:377–385
Magloczky Z et al (2000) Changes in the distribution and connectivity of interneurons in the epileptic human dentate gyrus. Neuroscience 96:7–25
Magloczky Z et al (2010) Dynamic changes of CB1-receptor expression in hippocampi of epileptic mice and humans. Epilepsia 51(Suppl 3):115–120
Margerison JH, Corsellis JAN (1966) Epilepsy and the temporal lobe. Brain 89:499–530
Martin JL, Sloviter RS (2001) Focal inhibitory interneuron loss and principal cell hyperexcitability in the rat hippocampus after microinjection of a neurotoxic conjugate of saporin and a peptidase-resistant analog of Substance P. J Comp Neurol 436:127–152
Mathern GW, Babb TL, Armstrong DL (1997a) Hippocampal Sclerosis. In: Epilepsy: A Comprehensive Textbook. Vol 13 J.J. Engel, T.A. Pedley, ed. Lipincott-Raven, Philadelphia, pp 133–155
Mathern GW et al (1997b) Human hippocampal AMPA and NMDA mRNA levels in temporal lobe epilepsy patients. Brain 120:1937–1959
Mathern GW et al (1998) Altered hippocampal kainate-receptor mRNA levels in temporal lobe epilepsy patients. Neurobiol Dis 5:151–176
McNamara JO (1999) Emerging insights into the genesis of epilepsy. Nature 399:A15–A22
Mello LE et al (1993) Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia 34:985–995
Mody I et al (1995) GABAergic inhibition of granule cells and hilar neuronal synchrony following ischemia-induced hilar neuronal loss. Neuroscience 69:139–150
Munoz A et al (2007) Cation-chloride cotransporters and GABA-ergic innervation in the human epileptic hippocampus. Epilepsia 48:663–673
Okabe A et al (2003) Changes in chloride homeostasis-regulating gene expressions in the rat hippocampus following amygdala kindling. Brain Res 990:221–226
Papp E et al (2008) Relationship between neuronal vulnerability and potassium-chloride cotransporter 2 immunoreactivity in hippocampus following transient forebrain ischemia. Neuroscience 154:677–689
Pathak HR et al (2007) Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci Off J Soc Neurosci 27:14012–14022
Payne JA (1997) Functional characterization of the neuronal-specific K–Cl cotransporter: implications for [K+]o regulation. Am J Physiol 273:C1516–C1525
Payne JA, Stevenson TJ, Donaldson LF (1996) Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J Biol Chem 271:16245–16252
Puskarjov M et al (2012) Activity-dependent cleavage of the K-Cl cotransporter KCC2 mediated by calcium-activated protease calpain. J Neurosci 32:11356–11364
Puskarjov M et al (2014) A variant of KCC2 from patients with febrile seizures impairs neuronal Cl− extrusion and dendritic spine formation. EMBO Rep 15:723–729
Reid KH et al (2001) The mRNA level of the potassium-chloride cotransporter KCC2 covaries with seizure susceptibility in inferior colliculus of the post-ischemic audiogenic seizure-prone rat. Neurosci Lett 308:29–32
Rivera C et al (1999) The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397:251–255
Rivera C et al (2002) BDNF-induced TrkB activation down-regulates the K+–Cl− cotransporter KCC2 and impairs neuronal Cl− extrusion. J Cell Biol 159:747–752
Robbins RJ et al (1991) A selective loss of somatostatin in the hippocampus of patients with temporal lobe epilepsy. Ann Neurol. 29:325–332
Sedmak G, Jovanov-Miloševic N, Puskarjov M, Ulamec M, Krušlin B, Kaila K, Judas M (2015) Developmental expression patterns of KCC2 and functionally associated molecules in the human brain (Cerebral Cortex, in press)
Seress L et al (2009) Survival of mossy cells of the hippocampal dentate gyrus in humans with mesial temporal lobe epilepsy Clinical article. J Neurosurg 111:1237–1247
Sloviter RS (1987) Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science 235:73–76
Smirnov S et al (1999) Pharmacological isolation of the synaptic and nonsynaptic components of the GABA-mediated biphasic response in rat CA1 hippocampal pyramidal cells. J Neurosci 19:9252–9260
Spencer DD, Spencer SS (1985) Surgery for epilepsy. Neurol Clin 3:313–330
Stein V, Nicoll RA (2003) GABA generates excitement. Neuron. 37:375–378
Stewart TH et al (2010) Chronic dysfunction of astrocytic inwardly rectifying K+ channels specific to the neocortical epileptic focus after fluid percussion injury in the rat. J Neurophysiol 104:3345–3360
Sutula T et al (1989) Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol 26:321–330
Swann JW et al (2000) Spine loss and other dendritic abnormalities in epilepsy. Hippocampus 10:617–625
Szabadics J et al (2006) Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311:233–235
Talos DM et al (2012) Altered inhibition in tuberous sclerosis and type IIb cortical dysplasia. Ann Neurol 71:539–551
Toth K et al (2007) Morphology and synaptic input of substance P receptor-immunoreactive interneurons in control and epileptic human hippocampus. Neuroscience 144:495–508
Toth K et al (2010) Loss and reorganization of calretinin-containing interneurons in the epileptic human hippocampus. Brain 133:2763–2777
Urban Z, Magloczky Z, Freund TF (2002) Calretinin-containing interneurons innervate both principal cells and interneurons in the CA1 region of the human hippocampus. Acta Biol Hung 53:205–220
Viitanen T et al (2010) The K+ -Cl cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol 588:1527–1540
Wake H et al (2007) Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. The Journal of neuroscience. 27:1642–1650
Williams JR et al (1999) The neuron-specific K-Cl cotransporter, KCC2. Antibody development and initial characterization of the protein. J Biol Chem 274:12656–12664
Wilson CL (1999) Neurophysiology of epileptic limbic pathways in intact human temporal lobe. In: The Epilepsies. Etiologies and prevention. Vol. P.K.a.H.O. Lüders, ed. Academic Press, San Diego, USA, pp 171–179
Wilson CL et al (1998) Paired pulse suppression and facilitation in human epileptogenic hippocampal formation. Epilepsy Res 31:211–230
Wittner L et al (2001) Preservation of perisomatic inhibitory input of granule cells in the epileptic human dentate gyrus. Neuroscience 108:587–600
Wittner L et al (2002) Synaptic reorganization of calbindin-positive neurons in the human hippocampal CA1 region in temporal lobe epilepsy. Neuroscience 115:961–978
Wittner L et al (2005) Surviving CA1 pyramidal cells receive intact perisomatic inhibitory input in the human epileptic hippocampus. Brain 128:138–152
Ylinen A et al (1991) Behavioural, electrophysiological and histopathological changes following sustained stimulation of the perforant pathway input to the hippocampus: effect of the NMDA receptor antagonist, CGP 39551. Brain Res 553:195–200
Zhang W et al (2009) Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci 29:14247–14256
Zhu ZQ et al (1997) Disproportionate loss of CA4 parvalbumin-immunoreactive interneurons in patients with Ammon’s horn sclerosis. J Neuropathol Exp Neurol 56:988–998
Acknowledgments
The authors are grateful to Drs. M. Palkovits, P. Sótonyi and Zs. Borostyánkői (Semmelweis University, Budapest) for providing control human tissue. We thank Mr. Gergő Botond for his helpful suggestions regarding Western blot experiments. The excellent technical assistance of Ms. E. Simon, K. Lengyel, Mr. Gy. Goda is also acknowledged. This study was supported by grants from OTKA 102802 Hungary, and NS030549NIH.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karlócai, M.R., Wittner, L., Tóth, K. et al. Enhanced expression of potassium-chloride cotransporter KCC2 in human temporal lobe epilepsy. Brain Struct Funct 221, 3601–3615 (2016). https://doi.org/10.1007/s00429-015-1122-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-015-1122-8