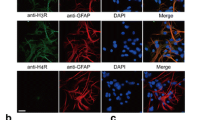
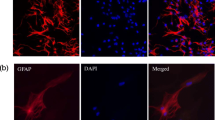
Abstract
Accumulation of β-amyloid (Aβ) protein within the brain is a neuropathological hallmark of Alzheimer’s disease (AD). One strategy to facilitate Aβ clearance from the brain is to promote Aβ catabolism. Matrix metalloproteinase-9 (MMP-9), a member of the family of Zn+2-containing endoproteases, known to be expressed and secreted by astrocytes, is capable of degrading Aβ. Histamine, a major aminergic brain neurotransmitter, stimulates the production of MMP-9 in keratinocytes through the histamine H1 receptor (H1R). In the present study, we show that histamine evokes a concentration- and calcium-dependent release of MMP-9 from human astrocytic U373 cells and primary cultures of human and rat astrocytes through the H1R subtype. Activation of H1R on astrocytes elevated intracellular levels of Ca2+ that was accompanied by time-dependent increases in MAP kinase p44/p42 and PKC. In-cell western blots revealed dose-dependent increases in both enzymes, confirming involvement of these signal transduction pathways. We next investigated the extent of recombinant human MMP-9 (rhMMP-9) proteolytic activity on soluble oligomeric Aβ (soAβ). Mass spectrometry demonstrated time-dependent cleavage of soAβ (20 μM), but not another amyloidogenic protein amylin, upon incubation with rhMMP-9 (100 nM) at 1, 4 and 17 h. Furthermore, Western blots showed a shift in soAβ equilibrium toward lower order, less toxic monomeric species. In conclusion, both MAPK p44/p42 and PKC pathways appear to be involved in histamine-upregulated MMP-9 release via H1Rs in astrocytes. Furthermore, MMP-9 appears to cleave soAβ into less toxic monomeric species. Given the key role of histamine in MMP-9 release, this neurotransmitter may serve as a potential therapeutic target for AD.







Similar content being viewed by others
References
Acharjee S, Zhu Y, Maingat F, Pardo C, Ballanyi K, Hollenberg MD, Power C (2011) Proteinase-activated receptor-1 mediates dorsal root ganglion neuronal degeneration in HIV/AIDS. Brain 134:3209–3221. doi:10.1093/brain/awr242
Akiyama H et al (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21:383–421
Arias-Montano JA, Berger V, Young JM (1994) Calcium-dependence of histamine- and carbachol-induced inositol phosphate formation in human U373 MG astrocytoma cells: comparison with HeLa cells and brain slices. Br J Pharmacol 111:598–608
Asahina M, Yoshiyama Y, Hattori T (2001) Expression of matrix metalloproteinase-9 and urinary-type plasminogen activator in Alzheimer’s disease brain. Clin Neuropathol 20:60–63
Asano K, Kanai KI, Suzaki H (2004) Suppressive activity of fexofenadine hydrochloride on metalloproteinase production from nasal fibroblasts in vitro. Clin Exp Allergy 34:1890–1898. doi:10.1111/j.1365-2222.2004.02127.x
Backstrom JR, Lim GP, Cullen MJ, Tokes ZA (1996) Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1-40). J Neurosci 16:7910–7919
Bakker RA, Timmerman H, Leurs R (2002) Histamine receptors: specific ligands, receptor biochemistry, and signal transduction. Clin Allergy Immunol 17:27–64
Berman NE et al (1999) Microglial activation and neurological symptoms in the SIV model of NeuroAIDS: association of MHC-II and MMP-9 expression with behavioral deficits and evoked potential changes. Neurobiol Dis 6:486–498. doi:10.1006/nbdi.1999.0261
Breunig E, Michel K, Zeller F, Seidl S, Weyhern CW, Schemann M (2007) Histamine excites neurones in the human submucous plexus through activation of H1, H2, H3 and H4 receptors. J Physiol 583:731–742. doi:10.1113/jphysiol.2007.139352
Bruno MA, Leon WC, Fragoso G, Mushynski WE, Almazan G, Cuello AC (2009) Amyloid beta-induced nerve growth factor dysmetabolism in Alzheimer disease. J Neuropathol Exp Neurol 68:857–869. doi:10.1097/NEN.0b013e3181aed9e6
Bu X, Zhao C, Dai X (2011) Involvement of COX-2/PGE(2) pathway in the upregulation of MMP-9 expression in pancreatic cancer. Gastroenterol Res Pract 2011:214269. doi:10.1155/2011/214269
Carman-Krzan M, Vige X, Wise BC (1991) Regulation by interleukin-1 of nerve growth factor secretion and nerve growth factor mRNA expression in rat primary astroglial cultures. J Neurochem 56:636–643
Cheng CY, Kuo CT, Lin CC, Hsieh HL, Yang CM (2010) IL-1beta induces expression of matrix metalloproteinase-9 and cell migration via a c-Src-dependent, growth factor receptor transactivation in A549 cells. Br J Pharmacol 160:1595–1610. doi:10.1111/j.1476-5381.2010.00858.x
Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, LaDu MJ (2002) Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem 277:32046–32053. doi:10.1074/jbc.M201750200
Deb S, Gottschall PE (1996) Increased production of matrix metalloproteinases in enriched astrocyte and mixed hippocampal cultures treated with beta-amyloid peptides. J Neurochem 66:1641–1647
Ding X, MacTavish D, Kar S, Jhamandas JH (2006) Galanin attenuates beta-amyloid (Abeta) toxicity in rat cholinergic basal forebrain neurons. Neurobiol Dis 21:413–420. doi:10.1016/j.nbd.2005.08.016
Doyle JL, Haas TL (2009) Differential role of beta-catenin in VEGF and histamine-induced MMP-2 production in microvascular endothelial cells. J Cell Biochem 107:272–283. doi:10.1002/jcb.22123
Gschwandtner M, Purwar R, Wittmann M, Baumer W, Kietzmann M, Werfel T, Gutzmer R (2008) Histamine upregulates keratinocyte MMP-9 production via the histamine H1 receptor. J Invest Dermatol 128:2783–2791. doi:10.1038/jid.2008.153
Haas H, Panula P (2003) The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci 4:121–130. doi:10.1038/nrn1034nrn1034
Hao F, Tan M, Xu X, Cui MZ (2008) Histamine induces Egr-1 expression in human aortic endothelial cells via the H1 receptor-mediated protein kinase Cdelta-dependent ERK activation pathway. J Biol Chem 283:26928–26936. doi:10.1074/jbc.M803071200
Hu WW, Chen Z (2012) Role of histamine and its receptors in cerebral ischemia. ACS Chem Neurosci 3:238–247. doi:10.1021/cn200126p
Inagaki N, Fukui H, Taguchi Y, Wang NP, Yamatodani A, Wada H (1989) Characterization of histamine H1-receptors on astrocytes in primary culture: [3H]mepyramine binding studies. Eur J Pharmacol 173:43–51. doi:10.1016/0014-2999(89)90007-1
Jhamandas JH, MacTavish D (2004) Antagonist of the amylin receptor blocks beta-amyloid toxicity in rat cholinergic basal forebrain neurons. J Neurosci 24:5579–5584. doi:10.1523/JNEUROSCI.1051-04.2004
Jones GJ et al (2007) HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci 27:3703–3711. doi:10.1523/JNEUROSCI.5522-06.2007
Juric DM, Mele T, Carman-Krzan M (2011) Involvement of histaminergic receptor mechanisms in the stimulation of NT-3 synthesis in astrocytes. Neuropharmacology 60:1309–1317. doi:10.1016/j.neuropharm.2011.01.019
Koistinaho M et al (2004) Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med 10:719–726. doi:10.1038/nm1058nm1058
Komatsu K, Nakanishi Y, Nemoto N, Hori T, Sawada T, Kobayashi M (2004) Expression and quantitative analysis of matrix metalloproteinase-2 and -9 in human gliomas. Brain Tumor Pathol 21:105–112
Lacor PN et al (2007) Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci 27:796–807. doi:10.1523/JNEUROSCI.3501-06.2007
Leissring MA (2006) Proteolytic degradation of the amyloid beta-protein: the forgotten side of Alzheimer’s disease. Curr Alzheimer Res 3:431–435
Li SF, Wu MN, Wang XH, Yuan L, Yang D, Qi JS (2011) Requirement of alpha7 nicotinic acetylcholine receptors for amyloid beta protein-induced depression of hippocampal long-term potentiation in CA1 region of rats in vivo. Synapse 65:1136–1143. doi:10.1002/syn.20951
Lipnik-Stanglj M, Carman-Krzan M (2000) The effects of histamine and interleukin-6 on NGF release from cortical astrocytes in primary culture. Pflugers Arch 440:R99–R100
Lorenzl S, Buerger K, Hampel H, Beal MF (2008) Profiles of matrix metalloproteinases and their inhibitors in plasma of patients with dementia. Int Psychogeriatr 20:67–76. doi:10.1017/S1041610207005790
Maier CM, Hsieh L, Yu F, Bracci P, Chan PH (2004) Matrix metalloproteinase-9 and myeloperoxidase expression: quantitative analysis by antigen immunohistochemistry in a model of transient focal cerebral ischemia. Stroke 35:1169–1174. doi:10.1161/01.STR.0000125861.55804.f2
Matsubara M, Tamura T, Ohmori K, Hasegawa K (2005) Histamine H1 receptor antagonist blocks histamine-induced proinflammatory cytokine production through inhibition of Ca2+-dependent protein kinase C, Raf/MEK/ERK and IKK/I kappa B/NF-kappa B signal cascades. Biochem Pharmacol 69:433–449. doi:10.1016/j.bcp.2004.10.006
Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S (2008) Abeta-degrading enzymes in Alzheimer’s disease. Brain Pathol 18:240–252. doi:10.1111/j.1750-3639.2008.00132.x
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC (2004) Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging 25:663–674. doi:10.1016/j.neurobiolaging.2004.01.007
Page-McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8:221–233. doi:10.1038/nrm2125
Panula P, Rinne J, Kuokkanen K, Eriksson KS, Sallmen T, Kalimo H, Relja M (1998) Neuronal histamine deficit in Alzheimer’s disease. Neuroscience 82:993–997
Rosenberg GA (2009) Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 8:205–216. doi:10.1016/S1474-4422(09)70016-X
Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K (2006) High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci 26:11870–11880. doi:10.1523/JNEUROSCI.3357-06.2006
Saido TC (1998) Alzheimer’s disease as proteolytic disorders: anabolism and catabolism of beta-amyloid. Neurobiol Aging 19:S69–S75
Saito T, Takaki Y, Iwata N, Trojanowski J, Saido TC (2003) Alzheimer’s disease, neuropeptides, neuropeptidase, and amyloid-beta peptide metabolism. Sci Aging Knowledge Environ 2003:PE1
Selkoe DJ (2000) Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann NY Acad Sci 924:17–25
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298:789–791. doi:10.1126/science.1074069
Stine WB Jr, Dahlgren KN, Krafft GA, LaDu MJ (2003) In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem 278:11612–11622. doi:10.1074/jbc.M210207200
Tetlow LC, Woolley DE (2002) Histamine stimulates matrix metalloproteinase-3 and -13 production by human articular chondrocytes in vitro. Ann Rheum Dis 61:737–740
Turner AJ, Fisk L, Nalivaeva NN (2004) Targeting amyloid-degrading enzymes as therapeutic strategies in neurodegeneration. Ann NY Acad Sci 1035:1–20. doi:10.1196/annals.1332.001
Vehmas AK, Kawas CH, Stewart WF, Troncoso JC (2003) Immune reactive cells in senile plaques and cognitive decline in Alzheimer’s disease. Neurobiol Aging 24:321–331
Wegiel J, Wang KC, Tarnawski M, Lach B (2000) Microglia cells are the driving force in fibrillar plaque formation, whereas astrocytes are a leading factor in plague degradation. Acta Neuropathol 100:356–364
Wyss-Coray T, Mucke L (2002) Inflammation in neurodegenerative disease—a double-edged sword. Neuron 35:419–432
Wyss-Coray T et al (2003) Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med 9:453–457. doi:10.1038/nm838
Yan P et al (2006) Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J Biol Chem 281:24566–24574. doi:10.1074/jbc.M602440200
Yang BC, Chen RF, Chio CC, Chang WC, Lin MT, Lin SJ (1998) Effects of insulin on protein phosphorylation and protein kinase C activity in human malignant gliomas. Proc Natl Sci Counc Repub China B 22:22–30
Yin KJ et al (2006) Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci 26:10939–10948. doi:10.1523/JNEUROSCI.2085-06.2006
Yoo HG et al (2002) IL-1beta induces MMP-9 via reactive oxygen species and NF-kappaB in murine macrophage RAW 264.7 cells. Biochem Biophys Res Commun 298:251–256
Acknowledgments
We thank the Pulmonary Research Group at the University of Alberta for access to ICW instrumentation and technical assistance with experiments utilizing this technique. We also thank Dr. K. Ballanyi for assistance with calcium imaging experiments. This research was supported by grants from the University Hospital Foundation (FoMD/UHF Medical Grants competition) and Canadian Institutes of Health Research (CIHR MOP 93601 to JHJ).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
429_2015_1007_MOESM1_ESM.eps
Supplementary Fig. 1 Gelatin zymogram showing histamine-induced upregulation of Pro-MMP-9 production was not blocked in a concentration-dependent manner following 24 h pre-incubation with H2R (Ranitidine HCl) and H3R (Clobenpropit HCl) antagonists (2.5–100 µM dose range) (EPS 4463 kb)
Rights and permissions
About this article
Cite this article
Patel, A., Vasanthan, V., Fu, W. et al. Histamine induces the production of matrix metalloproteinase-9 in human astrocytic cultures via H1-receptor subtype. Brain Struct Funct 221, 1845–1860 (2016). https://doi.org/10.1007/s00429-015-1007-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-015-1007-x