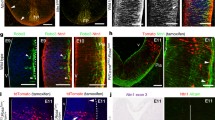
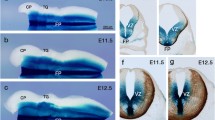
Abstract
The retroflex tract contains medial habenula efferents that target the hindbrain interpeduncular complex and surrounding areas. This tract displays a singular course. Initially, habenular axons extend ventralwards in front of the pretectum until they reach the basal plate. Next, they avoid crossing the local floor plate, sharply changing course caudalwards (the retroflexion alluded by the tract name) and navigate strictly antero-posteriorly across basal pretectum, midbrain and isthmus. Once they reach rhombomere 1, the habenular axons criss-cross the floor plate several times within the interpeduncular nuclear complex as they innervate it. Here we described the timing and details of growth phenomena as these axons navigate to their target. The first dorsoventral course apparently obeys Ntn1 attraction. We checked the role of local floor plate signaling in the decision to avoid the thalamic floor plate and bend caudalwards. Analyzing the altered floor and basal plates of Gli2 knockout mice, we found a contralateral projection of most habenular axons, plus ulterior bizarre navigation rostralwards. This crossing phenotype was due to a reduced expression of Slit repulsive cues, suggesting involvement of the floor-derived Robo-Slit system in the normal guidance of this tract. Using Slit and Robo mutant mice, open neural tube and co-culture assays, we determined that Robo1-Slit2 interaction is specifically required for impeding that medial habenular axons cross the thalamic floor plate. This pathfinding mechanism is essential to establish the functionally important habenulo-interpeduncular connection.







Similar content being viewed by others
References
Aizawa H, Bianco IH, Hamaoka T, Miyashita T, Uemura O, Concha ML, Russell C, Wilson SW, Okamoto H (2005) Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Nat Rev Neurosci 13:832–834
Andres KH, von Düring M, Veh RW (1999) Subnuclear organization of the rat habenular complexes. J Comp Neurol 407:130–150
Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JLR, Tessier-Lavigne M (2002) Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron 33:233–248
Baldock RA, Bard JB, Burger A, Burton N, Christiansen J, Feng G, Hill B, Houghton D, Kaufman M, Rao J, Sharpe J, Ross A, Stevenson P, Venka- taraman S, Waterhouse A, Yang Y, Davidson DR (2003) EMAP and EMAGE: a framework for understanding spatially organized data. Neuroinformatics 1:309–325
Beretta CA, Dross N, Guiterrez-Triana JA, Ryu S, Carl M (2012) Habenula circuit development: past, present, and future. Front Neurosci 6:51
Bianco IH, Wilson SW (2009) The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci 364:1005–1020
Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T (1999) Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96:795–806
Camurri L, Mambetisaeva E, Sundaresan V (2004) Rig-1 a new member of Robo family genes exhibits distinct pattern of expression during mouse development. Gene Expr Patterns 4:99–103
Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M (2003) The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell 113:11–23
Christiansen JH, Yang Y, Venkataraman S, Richardson L, Stevenson P, Burton N, Baldock RA, Davidson (2006) EMAGE: a spatial database of gene expression patterns during mouse embryo development. Nucleic Acids Res 34(Database issue):D637–D641
Colamarino SA, Tessier-Lavigne M (1995) The role of the floor plate in axon guidance. Annu Rev Neurosci 18:497–529
Concha ML, Wilson SW (2001) Asymmetry in the epithalamus of vertebrates. J Anat 199:63–84
Devine CA, Key B (2008) Robo-Slit interactions regulate longitudinal axon pathfinding in the embryonic vertebrate brain. Dev Biol 313:371–383
Di Meglio T, Nguyen-Ba-Charvet KT, Tessier-Lavigne M, Sotelo C, Chédotal A (2008) Molecular mechanisms controlling midline crossing by precerebellar neurons. J Neurosci 28:6285–6294
Ding Q, Motoyama J, Gasca S, Mo R, Sasaki H, Rossant J, Hui CC (1998) Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development 125:2533–2543
Dong HW (2008) The allen reference atlas: a digital color brain atlas of the C57BL/6J male mouse. The Allen Institute for Brain Science/Wiley & Sons, London
Farmer WT, Altick AL, Nural HF, Dugan JP, Kidd T, Charron F, Mastick GS (2008) Pioneer longitudinal axons navigate using floor plate and Slit/Robo signals. Development 135:3643–3653
Funato H, Saito-Nakazato Y, Takahashi H (2000) Axonal growth from the habenular nucleus along the neuromere boundary region of the diencephalon is regulated by semaphorin 3F and netrin-1. Mol Cell Neurosci 16:206–220
Geisler M, Trimble M (2008) The lateral habenula: no longer neglected. CNS Spectr 13:484–489
Grieshammer U, Ma Le, Plump AS, Wang F, Tessier-Lavigne M, Martin GR (2004) SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell 6:709–717
Herrick CJ (1910) The morphology of the forebrain in amphibia and reptilia. J Comp Neurol Psychol 20:413–547
Hong S, Hikosaka O (2008) The globus pallidus sends reward-related signals to the lateral habenula. Neuron 60:720–729
Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O (2011) Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci 31:11457–11471
Kadison SR, Murakami F, Matise MP, Kaprielian Z (2006) The role of floor plate contact in the elaboration of contralateral commissural projections within the embryonic mouse spinal cord. Dev Bio 296:499–513
Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguch Y, Sretavan DW, Giger RJ, Kolodkin AL (2004) Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron 44:961–975
Kaprielian Z, Runko E, Imondi R (2001) Axon guidance at the midline choice point. Dev Dyn 221:154–181
Kastenhuber E, Kern U, Bonkowsky JL, Chien C, Driever W, Schweitzer J (2009) Netrin-DCC, Robo-Slit, and heparan sulfate proteoglycans coordinate lateral positioning of longitudinal dopaminergic diencephalospinal axons. J Neurosci 9:8914–8926
Kennedy TE, Serafini T, Torre La, de JR, Tessier-Lavigne M (1994) Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78:425–435
Kim M, Roesener AP, Mendonca PRF, Mastick GS (2011) Robo1 and Robo2 have distinct roles in pioneer longitudinal axon guidance. Dev Biol 358:181–188
Klemm WR (2004) Habenular and interpeduncularis nuclei: shared components in multiple-function networks. Med Sci Monit 10:RA261–RA273
Kuan YS, Gamse JT, Schreiber AM, Halpern ME (2007) Selective asymmetry in a conserved forebrain to midbrain projection. J Exp Zool B Mol Dev Evol 308:669–678
Kuwajima T, Sitko AA, Bhansali P, Jurgens C, Guido W, Mason C (2013) ClearT: a detergent- and solvent-free clearin method for neuronal and non-neuronal Tissue. Development 140:1364–1368
Lecourtier L, Kelly PH (2007) A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev 31:658–672
Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, Ornitz DM, Wu JY, Rao Y (1999) Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell 96:807–818
Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M (2004) Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron 42:213–223
López-Bendito G, Flames N, Ma L, Fouquet C, Di Meglio T, Chédotal A, Tessier-Lavigne M, Marín O (2007) Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J Neurosci 27:3395–3407
Marillat V, Sabatier C, Failli V, Matsunaga E, Sotelo C, Tessier-Lavigne M, Chédotal A (2004) Neuron 43:69–79
Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL (1998) Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development 125:2759–2770
Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner AL, Hui C (1997) Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development 124:113–123
Moreno-Bravo JA, Perez-Balaguer A, Martinez-Lopez JE, Aroca P, Puelles L, Martínez S, Puelles E (2014) Role of Shh in the development of molecularly characterized tegmental nuclei in mouse rhombomere 1. Brain Struc Funct 219:777–792
Morgane PJ, Galler JR, Mokler DJ (2005) A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol 75:143–160
Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M (2002) Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron 33:219–232
Puelles L, Rubenstein JLR (2003) Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci 26:469–476
Puelles E, Martinez-de-la-Torre M, Watson C, Puelles L (2012) Midbrain. In: Watson C, Paxinos G, Puelles L (eds) Chapter 10 in the mouse nervous system. Academic Press/Elsevier, New York, pp 337–359
Quina LA, Pak W, Lanier J, Banwait P, Gratwick K, Liu Y, Velasquez T, O’Leary DDM, Goulding M, Turner EE (2005) Brn3a-expressing retinal ganglion cells project specifically to thalamocortical and collicular visual pathways. J Neurosci 25:11595–11604
Quina LA, Wang S, Ng L, Turner EE (2009) Brn3a and Nurr1 mediate a gene regulatory pathway for habenula development. J Neurosci 29:14309–14322
Ramón y Cajal (1909) Histologie du système nerveux de l’homme & des vertébrés, vol I. Re-edition by CSIC, Madrid, 1955
Ricaño-Cornejo I, Altick AL, García-Peña CM, Nural HF, Echevarria D, Miquelajáuregui A, Mastick GS, Varela-Echavarría A (2011) Slit-Robo signals regulate pioneer axon pathfinding of the tract of the postoptic commissure in the mammalian forebrain. J Neurosci Res 89:1531–1541
Sahay A, Molliver ME, Ginty DD, Kolodkin AL (2003) Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J Neurosci 23:6671–6680
Schmidt ER, Brignani S, Adolfs Y, Lemstra S, Demmers J, Vidaki M, Donahoo AL, Lilleväli K, Vasar E, Richards LJ, Karagogeos D, Kolk Sm, Pasterkamp Rj (2014) Subdomain-mediated axon-axon signaling and chemoattraction cooperate to regulate afferent innervation of the lateral habenula. Neuron 83:372–387
Sutherland RJ (1982) The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev 6:1–13
Tessier-Lavigne M, Goodman CS (1996) The molecular biology of axon guidance. Science 274:1123–1133
Viswanath H, Carter AQ, Baldwin PR, Molfese DL, Salas R (2014) The medial habenula: still neglected. Front Hum Neurosci 7:931
Zhang C, Gao J, Zhang H, Sun L, Peng G (2012) Robo2–slit and Dcc–netrin1 coordinate neuron axonal pathfinding within the embryonic axon tracts. J Neurosci 32:12589–12602
Zheng W, Geng AQ, Li PF, Wang Y, Yuan XD (2012) Robo4 regulates the radial migration of newborn neurons in developing neocortex. Cereb Cortex 22:2587–2601
Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M (2000) Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell 102:363–375
Acknowledgments
Work supported by ‘‘Ministerio de Economía y Competitividad’’ BFU2010-16548 and BFU2013-48230-P (FEDER Fonds) to E. Puelles, BFU2012-34298 to G. Lopez-Bendito; Consolider Grant (CSD2007-00023) and European commission (EUCOMMTOOLS, contract 261492) to S.M. J.A. Moreno-Bravo was supported by the Predoctoral Program of the ‘‘Consejo Superior de Investigaciones Científicas-Junta de Ampliación de Estudios’’, co-financed by the European Social Fund. The Instituto de Neurociencias is a “Centre of Excellence Severo Ochoa”. Funding was also provided to G.S. Mastick by NIH grants R21NS077169, with core facility support by NIH COBREs 1 P20 RR024210 and 1 P20 GM103650, and the Nevada INBRE 8 P20 GM103440-11. We thank E. Turner for kindly providing the Pou4f1-TauLacZ transgenic line and J. Pasterkamp for kindly providing the Slit2 mutant embryos. We are also grateful to O. Marín for providing the Robo1, Robo2, Slit1, Slit2, Slit3 probes; O. Reiner for providing the Netrin1 probe; M. Frohman for proving Gbx2, A. McMahon for Shh and J.L Rubenstein for Dlx2 probe. M. Tessier-Lavigne for providing the Slit1 functional construct and A. Nieto for providing the PCX-GFP plasmid. We thank E. Leyva-Diaz, R. Susin and C. Merino from Lopez-Bendito lab for helping us in the generation and genotyping of the Robo transgenic embryos. We are also grateful with E. Domiguez and A. Sempere for statistical advice, and L. Puelles and the staff from the S. Martínez lab, especially D. Echevarría, for helpful discussions and comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online resource legends:

429_2014_932_MOESM2_ESM.jpg
ESM2: Open neural tube technic (ONT). (a, b) Schematic design of the ONTs, we dissected the neural tube and cut the rostral forebrain and caudal hindbrain out. The remaining neural tube was opened like a book through the roof plate where we maintained the adjacent territories involved in the guidance of the rft. (c) X-gal stained-E12.5 ONT in which the Pou4f1 positive neurons and their axons are labeled, habenula and the rft among them. (d, e) In situ hybridization for Gbx2 (d) and Shh (e) displaying the location of the thalamus and the basal and floor plate, respectively. We demonstrated that the ONTs allow us to study the course of the rtf due to the maintenance of the positional relations of the territories involved. Abbreviations: ap, alar plate; bp, basal plate; fp, floor plate; Hb, Habenula; Ist, Isthmus; Mb, Midbrain; p1, prosomere 1; p2, prosomere 2; p3, prosomere 3; pc, posterior commissure; r1, rhombomere 1; rft, retroflex tract; rp, roof plate; Th, Thalamus; zli, zona limitans intrathalamica. Scale bar: 200 μm. (JPEG 1154 kb)

429_2014_932_MOESM3_ESM.jpg
ESM3: Phenotype of the rft and neural territories in Gli2 mutant. Medial (a, c, e) and lateral (b, d, f) sagittal paraffin sections from an E18.5 Gli2 mutant brain. (a-b) Immunostaining against DCC reveals the rostral navigation of the rft in the midline (see arrow in a). (b) In a lateral section, the dorsoventral trajectory is showed. (c-f) In situ hybridization for Gbx2 (c, d) and Dlx2 (e, f) displaying the thalamus, and the hypothalamus–prethalamus, respectively. Abbreviations: Cb, Cerebellum; Hb, habenula; Hyp, Hypothalamus; Mb, Midbrain; pc, posterior commissure; PTh, Prethalamus; rft, retroflex tract; Th, Thalamus. Scale bar: 200 μm (JPEG 1884 kb)

429_2014_932_MOESM5_ESM.jpg
ESM5: Robo3 and Robo4 expression pattern in the habenula. (a-b) In situ hybridization for Robo3 (a, a’) and Robo4 (b, b’) in Hb paraffin coronal sections of an E12.5 (a, b) and E18.5 (a’, b’) embryo. Robo3 is strongly expressed in the mHb and Robo4 is not expressed in this region. The arrow in b shows the Robo4 expression in the cortex. The dotted line indicates the location of the habenular complex. Abbreviations: Hb, habenula; lHb, lateral habenula; mHb, medial habenula. Scale bar: 200 μm (JPEG 514 kb)
Rights and permissions
About this article
Cite this article
Moreno-Bravo, J.A., Martinez-Lopez, J.E., Madrigal, M.P. et al. Developmental guidance of the retroflex tract at its bending point involves Robo1-Slit2-mediated floor plate repulsion. Brain Struct Funct 221, 665–678 (2016). https://doi.org/10.1007/s00429-014-0932-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0932-4