Abstract
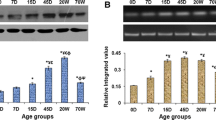
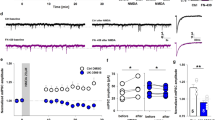
Age-related molecular changes in the synapse can cause plasticity decline. We found an impairment of experience-dependent cortical plasticity is induced by short lasting sensory conditioning in aged mice. However, extending the training procedure from 3 to 7 days triggered plasticity in the aged cortex of the same range as in young mice. Additionally, GABAergic markers (GABA, GAD67, VGAT) in young and aged groups that showed the plastic changes were upregulated. This effect was absent in the aged group with impaired plasticity, while the expression of Vglut1 increased in all trained groups. This may reflect the inefficiency of inhibitory mechanisms in the aging brain used to control increased excitation after training and to shape proper signal to noise ratio, which is essential for appropriate stimuli processing. HPLC analysis showed that the glutamate/GABA ratio was significantly reduced in aged animals due to a significant decrease in glutamate level. We also observed a decreased expression of several presynaptic markers involved in excitatory (vesicular glutamate transporter-vglut2) and inhibitory (glutamic acid decarboxylase-GAD67, vesicular GABA transporter VGAT) transmission in the aged barrel cortex. These changes may weaken the plasticity potential of neurons and impede the experience-dependent reorganization of cortical connections. We suggest that the imbalance toward inhibition resulting from a decrease of glutamate content in the aging cerebral cortex, together with GABAergic system ineffectiveness in upregulating GABA level after sensory training, contributes to the impairment of learning-dependent cortical plasticity.





Similar content being viewed by others
References
Akopian G, Walsh JP (2006) Pre- and postsynaptic contributions to age-related alterations in corticostriatal synaptic plasticity. Synapse 60:223–238
Benali A, Weiler E, Benali Y, Dinse HR, Eysel UT (2008) Excitation and inhibition jointly regulate cortical reorganization in adult rats. J Neurosci 28(47):12284–12293
Burianova J, Ouda L, Profant O, Syka J (2009) Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol 44:161–169
Burke SN, Barnes CA (2006) Neural plasticity in the ageing brain. Nat Rev Neurosci 7:30–40
Canas PM, Duarte JM, Rodrigues RJ, Kofalvi A, Cunha RA (2009) Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol Aging 30:1877–1884
Cheetham CE, Hammond MS, Edwards CE, Finnerty GT (2007) Sensory experience alters cortical connectivity and synaptic function site specifically. J Neurosci 27:3456–3465
Chen ZJ, He Y, Rosa-Neto P, Gong G, Evans AC (2011) Age-related alterations in the modular organization of structural cortical network by using cortical thickness from MRI. Neuroimage 56:235–245
Cheng XR, Yang Y, Zhou WX, Zhang YX (2011) Expression of VGLUTs contributes to degeneration and acquisition of learning and memory. Neurobiol Learn Mem 3:361–375
David-Jurgens M, Churs L, Berkefeld T, Zepka RF, Dinse HR (2008) Differential effects of aging on fore- and hindpaw maps of rat somatosensory cortex. PLoS One 3:e3399
De Gois S, Shäfer MK-H, Defamie N, Chen CH, Ricci A, Weihe E, Varoqui H, Erickson JD (2005) Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci 25:7121–7133
Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ (2006) The aging hippocampus: a multi-level analysis in the rat. Neuroscience 139:1173–1185
Dubroff JG, Stevens RT, Hitt J, Maier DL, McCasland JS, Hodge CJ (2005) Use-dependent plasticity in barrel cortex: intrinsic signal imaging reveals functional expansion of spared whisker representation into adjacent deprived columns. Somatosens Mot Res 22:25–35
Foster TC, Barnes CA, Rao G, McNaughton BL (1991) Increase in perforant path quantal size in aged F-344 rats. Neurobiol Aging 12:441–448
Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB (2013) Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage 78:75–82
Gierdalski M, Jablonska B, Siucinska E, Lech M, Skibinska A, Kossut M (2001) Rapid regulation of GAD67 mRNA and protein level in cortical neurons after sensory learning. Cereb Cortex 11:806–815
Godde B, Berkefeld T, David-Jurgens M, Dinse HR (2002) Age-related changes in primary somatosensory cortex of rats: evidence for parallel degenerative and plastic-adaptive processes. Neurosci Biobehav Rev 26:743–752
Goto N, Yoshimura R, Moriya J, Kakeda S, Hayashi K, Ueda N, Ikenouchi-Sugita A, Umene-Nakano W, Oonari N, Korogi Y, Nakamura J (2010) Critical examination of a correlation between brain gamma-aminobutyric acid (GABA) concentrations and a personality trait of extroversion in healthy volunteers as measured by a 3 Tesla proton magnetic resonance spectroscopy study. Psychiatry Res 182:53–57
Hickmott P, Dinse H (2012) Effects of aging on properties of the local circuit in rat primary somatosensory cortex (S1) in vitro. Cereb Cortex. doi:10.1093/cercor/bhs248
Hof PR, Morrison JH (2004) The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci 27:607–613
Ishikawa T, Sahara Y, Takahashi T (2002) A single packet of transmitter does not saturate postsynaptic glutamate receptors. Neuron 34:613–621
Itzev D, Lolova I, Lolov S, Usunoff KG (2001) Age-related changes in the synapses of the rat’s neostriatum. Arch Physiol Biochem 109:80–89
Jasinska M, Siucinska E, Cybulska-Klosowicz A, Pyza E, Furness DN, Kossut M (2010) Rapid, learning-induced inhibitory synaptogenesis in murine barrel field. J Neurosci 30:1176–1184
Kumar A, Foster TC (2007) Neurophysiology of old neurons and synapses. In: Riddle DR (ed) Brain aging: models, methods, and mechanisms, Chap 10. CRC Press, Boca Raton
Lau CG, Murthy VN (2012) Activity-dependent regulation of inhibition via GAD67. J Neurosci 32:8521–8531
Lehmann K, Schmidt KF, Löwel S (2012) Vision and visual plasticity in ageing mice. Restor Neurol Neurosci 30:161–178
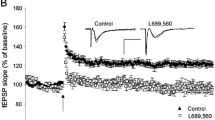
Liguz-Lecznar M, Siucinska E, Zakrzewska R, Kossut M (2011) Impairment of experience-dependent cortical plasticity in aged mice. Neurobiol Aging 32:1896–1905
Luebke JI, Chang YM, Moore TL, Rosene DL (2004) Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience 125:277–288
Magnusson KR, Cotman CW (1993) Age-related changes in excitatory amino acid receptors in two mouse strains. Neurobiol Aging 14:197–206
Majdi M, Ribeiro-da-Silva A, Cuello AC (2007) Cognitive impairment and transmitter-specific pre- and postsynaptic changes in the rat cerebral cortex during ageing. Eur J Neurosci 26:3583–3596
Marczynski TJ (1998) GABAergic deafferentation hypothesis of brain aging and Alzheimer’s disease revisited. Brain Res Bull 45:341–379
Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, Buist A, Cik M, van der Spek P, Kass S, Meert T, D’Hooge R, Rosenmund C, Hampson RM (2006) Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci 26:12055–12066
Navone F, Jahn R, Di Gioia G, Stukenbrok H, Greengard P, De Camilli P (1986) Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol 103:2511–2527
Paullus JR, Hickmott PW (2011) Diverse excitatory and inhibitory synaptic plasticity outcomes in complex horizontal circuits near a functional border of adult neocortex. Brain Res 1416:10–25
Peinemann A, Lehner C, Conrad B, Siebner HR (2001) Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett 313:33–36
Pellicciari MC, Miniussi C, Rossini PM, De Gennaro L (2009) Increased cortical plasticity in the elderly: changes in the somatosensory cortex after paired associative stimulation. Neuroscience 163:266–276
Poe BH, Linville C, Brunso-Bechtold J (2001) Age-related decline of presumptive inhibitory synapses in the sensorimotor cortex as revealed by the physical disector. J Comp Neurol 439:65–72
Rosenzweig ES, Barnes CA (2003) Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol 69:143–179
Rosenzweig ES, Rao G, McNaughton BL, Barnes CA (1997) Role of temporal summation in age-related long-term potentiation-induction deficits. Hippocampus 7:549–558
Rowley HL, Martin KF, Marsden CA (1995) Determination of in vivo amino acid neurotransmitters by high-performance liquid chromatography with o-phthalaldehyde-sulphite derivatisation. J Neurosci Methods 57:93–99
Ruano D, Araujo F, Revilla E, Vela J, Bergis O, Vitorica J (2000) GABAA and alpha-amino-3-hydroxy-5-methylsoxazole-4-propionate receptors are differentially affected by aging in the rat hippocampus. J Biol Chem 275:19585–19593
Sametsky EA, Disterhoft JF, Geinisman Y, Nicholson DA (2010) Synaptic strength and postsynaptically silent synapses through advanced aging in rat hippocampal CA1 pyramidal neurons. Neurobiol Aging 31:813–825
Saransaari P, Oja SS (1995) Age-related changes in the uptake and release of glutamate and aspartate in the mouse brain. Mech Ageing Dev 81:61–71
Segovia G, Mora F (2005) Effects of the metabotropic glutamate receptor agonist, ACPD, on the extracellular concentrations of GABA and acetylcholine in the prefrontal cortex of the rat during the normal process of aging. Brain Res Bull 65:11–16
Segovia G, Porras A, Del Arco A, Mora F (2001) Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev 122:1–29
Siucinska E, Kossut M (1996) Short-lasting classical conditioning induces reversible changes of representational maps of vibrissae in mouse SI cortex–a 2DG study. Cereb Cortex 6:506–513
Siucinska E, Kossut M, Stewart MG (1999) GABA immunoreactivity in mouse barrel field after aversive and appetitive classical conditioning training involving facial vibrissae. Brain Res 843:62–70
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916
Stanley EM, Fadel JR, Mott DD (2012) Interneuron loss reduces dendritic inhibition and GABA release in hippocampus of aged rats. Neurobiol Aging 33:e1–e13
Strominger RN, Woolsey TA (1987) Templates for locating the whisker area in fresh flattened mouse and rat cortex. J Neurosci Methods 22:113–118
Tokarski K, Urban-Ciecko J, Kossut M, Hess G (2007) Sensory learning-induced enhancement of inhibitory synaptic transmission in the barrel cortex of the mouse. Eur J Neurosci 26:134–141
Wallen-Mackenzie A, Wootz H, Englund H (2010) Genetic inactivation of the vesicular glutamate transporter 2 (VGLUT2) in the mouse: what have we learnt about functional glutamatergic neurotransmission? Ups J Med Sci 115:11–20
Wen JA, DeBlois MC, Barth AL (2013) Initiation, labile, and stabilization phases of experience-dependent plasticity at neocortical synapses. J Neurosci 33:8483–8493
Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G (2005) Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci 25:6221–6234
Wong TP, Marchese G, Casu MA, Ribeiro-da-Silva A, Cuello AC, De Koninck Y (2000) Loss of presynaptic and postsynaptic structures is accompanied by compensatory increase in action potential-dependent synaptic input to layer V neocortical pyramidal neurons in aged rats. J Neurosci 20:8596–8606
Wong TP, Marchese G, Casu MA, Ribeiro-da-Silva A, Cuello AC, De Koninck Y (2006) Imbalance towards inhibition as a substrate of aging-associated cognitive impairment. Neurosci Lett 397:64–68
Acknowledgments
This work was supported by statutable funds of Nencki Institute of Experimental Biology and by Ministry of Science and Education Grant No: NN301248639.
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liguz-Lecznar, M., Lehner, M., Kaliszewska, A. et al. Altered glutamate/GABA equilibrium in aged mice cortex influences cortical plasticity. Brain Struct Funct 220, 1681–1693 (2015). https://doi.org/10.1007/s00429-014-0752-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0752-6