Abstract
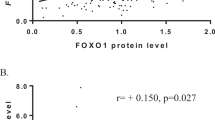
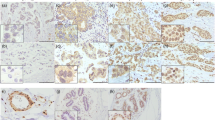
We studied the relationship between CD44 and Forkhead box P3 (FOXP3) gene expression in cell lines and breast carcinomas and their association with clinicopathological variables and patient outcome. We assessed messenger RNA (mRNA) expression of CD44 and FOXP3 by quantitative real-time PCR and determined the number of FOXP3+ Tregs by immunohistochemistry in 264 breast cancer specimens. CD44 was stimulated with hyaluronan treatment, and the accompanying changes in FOXP3 mRNA expression in breast cancer cell lines representing breast cancer subtype were assessed. We found that lower CD44 expression correlated with the presence of necrosis, lymph-vascular invasion, grade 3 tumors, and aggressive phenotype (HER2 and basal-like). FOXP3 mRNA correlated positively with CD44 mRNA expression and Treg content. Moreover, stimulation of CD44 expression by hyaluronan in cell lines increased FOXP3 expression, which supports that their regulation is associated. Survival analysis revealed that low CD44 expression is associated with higher frequency of recurrence. Our findings indicate that CD44 has a regulatory role in FOXP3 expression and is associated with good prognostic factors in breast cancer.



Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lonning PE, Borresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98:10869–10874
Orian-Rousseau V (2015) CD44 acts as a signaling platform controlling tumor progression and metastasis. Front Immunol 6:154
Louderbough JM, Schroeder JA (2011) Understanding the dual nature of CD44 in breast cancer progression. Mol Cancer Res 9:1573–1586
Foekens JA, Dall P, Klijn JG, Skroch-Angel P, Claassen CJ, Look MP, Ponta H, Van Putten WL, Herrlich P, Henzen-Logmans SC (1999) Prognostic value of CD44 variant expression in primary breast cancer. Int J Cancer 84:209–215
Berner HS, Suo Z, Risberg B, Villman K, Karlsson MG, Nesland JM (2003) Clinicopathological associations of CD44 mRNA and protein expression in primary breast carcinomas. Histopathology 42:546–554
Diaz LK, Zhou X, Wright ET, Cristofanilli M, Smith T, Yang Y, Sneige N, Sahin A, Gilcrease MZ (2005) CD44 expression is associated with increased survival in node-negative invasive breast carcinoma. Clin Cancer Res 11:3309–3314
Bankfalvi A, Terpe HJ, Breukelmann D, Bier B, Rempe D, Pschadka G, Krech R, Bocker W (1998) Gains and losses of CD44 expression during breast carcinogenesis and tumour progression. Histopathology 33:107–116
Joensuu H, Klemi PJ, Toikkanen S, Jalkanen S (1993) Glycoprotein CD44 expression and its association with survival in breast cancer. Am J Pathol 143:867–874
Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, Cheng C (2011) CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest 121:1064–1074
Zhou Z, Song X, Li B, Greene MI (2008) FOXP3 and its partners: structural and biochemical insights into the regulation of FOXP3 activity. Immunol Res 42:19–28
Firan M, Dhillon S, Estess P, Siegelman MH (2006) Suppressor activity and potency among regulatory T cells is discriminated by functionally active CD44. Blood 107:619–627
Bollyky PL, Falk BA, Long SA, Preisinger A, Braun KR, Wu RP, Evanko SP, Buckner JH, Wight TN, Nepom GT (2009) CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-beta. J Immunol 183:2232–2241
Zhang C, Xu Y, Hao Q, Wang S, Li H, Li J, Gao Y, Li M, Li W, Xue X, Wu S, Zhang Y, Zhang W (2015) FOXP3 suppresses breast cancer metastasis through downregulation of CD44. Int J Cancer 137:1279–1290
Martin F, Ladoire S, Mignot G, Apetoh L, Ghiringhelli F (2010) Human FOXP3 and cancer. Oncogene 29:4121–4129
Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grussel S, Sipos B, Grutzmann R, Pilarsky C, Ungefroren H, Saeger HD, Kloppel G, Kabelitz D, Kalthoff H (2007) Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res 67:8344–8350
Ebert LM, Tan BS, Browning J, Svobodova S, Russell SE, Kirkpatrick N, Gedye C, Moss D, Ng SP, MacGregor D, Davis ID, Cebon J, Chen W (2008) The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res 68:3001–3009
Karanikas V, Speletas M, Zamanakou M, Kalala F, Loules G, Kerenidi T, Barda AK, Gourgoulianis KI, Germenis AE (2008) Foxp3 expression in human cancer cells. J Transl Med 6:19
Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Menard S, Tagliabue E, Balsari A (2009) FOXP3 expression and overall survival in breast cancer. J Clin Oncol 27:1746–1752
Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, Liu Y, Wang Y, Liu X, Chan MW, Liu JQ, Love R, Liu CG, Godfrey V, Shen R, Huang TH, Yang T, Park BK, Wang CY, Zheng P, Liu Y (2007) FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell 129:1275–1286
Wang L, Liu R, Li W, Chen C, Katoh H, Chen GY, McNally B, Lin L, Zhou P, Zuo T, Cooney KA, Liu Y, Zheng P (2009) Somatic single hits inactivate the X-linked tumor suppressor FOXP3 in the prostate. Cancer Cell 16:336–346
Redpath M, Xu B, van Kempen LC, Spatz A (2011) The dual role of the X-linked FoxP3 gene in human cancers. Mol Oncol 5:156–163
Zuo T, Liu R, Zhang H, Chang X, Liu Y, Wang L, Zheng P, Liu Y (2007) FOXP3 is a novel transcriptional repressor for the breast cancer oncogene SKP2. J Clin Invest 117:3765–3773
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93:387–391
Peiro G, Adrover E, Sanchez-Tejada L, Lerma E, Planelles M, Sanchez-Paya J, Aranda FI, Giner D, Gutierrez-Avino FJ (2011) Increased insulin-like growth factor-1 receptor mRNA expression predicts poor survival in immunophenotypes of early breast carcinoma. Mod Pathol 24:201–208
Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, Senn HJ (2015) Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 26:1533–1546
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101:736–750
Lee S, Cho EY, Park YH, Ahn JS, Im YH (2013) Prognostic impact of FOXP3 expression in triple-negative breast cancer. Acta Oncologica (Stockholm, Sweden) 52:73–81
Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH (2006) Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 24:5373–5380
Holliday DL, Speirs V (2011) Choosing the right cell line for breast cancer research. Breast Cancer Res 13:215
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Haibe-Kains B, Desmedt C, Loi S, Culhane AC, Bontempi G, Quackenbush J, Sotiriou C (2012) A three-gene model to robustly identify breast cancer molecular subtypes. J Natl Cancer Inst 104:311–325
Friedrichs K, Franke F, Lisboa BW, Kugler G, Gille I, Terpe HJ, Holzel F, Maass H, Gunthert U (1995) CD44 isoforms correlate with cellular differentiation but not with prognosis in human breast cancer. Cancer Res 55:5424–5433
Olsson E, Honeth G, Bendahl PO, Saal LH, Gruvberger-Saal S, Ringner M, Vallon-Christersson J, Jonsson G, Holm K, Lovgren K, Ferno M, Grabau D, Borg A, Hegardt C (2011) CD44 isoforms are heterogeneously expressed in breast cancer and correlate with tumor subtypes and cancer stem cell markers. BMC Cancer 11:418
Dan T, Hewitt SM, Ohri N, Ly D, Soule BP, Smith SL, Matsuda K, Council C, Shankavaram U, Lippman ME, Mitchell JB, Camphausen K, Simone NL (2014) CD44 is prognostic for overall survival in the NCI randomized trial on breast conservation with 25 year follow-up. Breast Cancer Res Treat 143:11–18
Tokue Y, Matsumura Y, Katsumata N, Watanabe T, Tarin D, Kakizoe T (1998) CD44 variant isoform expression and breast cancer prognosis. Jpn J Cancer Res 89:283–290
Rys J, Kruczak A, Lackowska B, Jaszcz-Gruchala A, Brandys A, Stelmach A, Reinfuss M (2003) The role of CD44v3 expression in female breast carcinomas. Pol J Pathol 54:243–247
Negi LM, Talegaonkar S, Jaggi M, Ahmad FJ, Iqbal Z, Khar RK (2012) Role of CD44 in tumour progression and strategies for targeting. J Drug Target 20:561–573
Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, Earl HM, Poole CJ, Hiller L, Dunn JA, Bowden SJ, Twelves C, Bartlett JM, Mahmoud SM, Rakha E, Ellis IO, Liu S, Gao D, Nielsen TO, Pharoah PD, Caldas C (2014) Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol 25:1536–1543
Liu S, Foulkes WD, Leung S, Gao D, Lau S, Kos Z, Nielsen TO (2014) Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res 16:432
Lal A, Chan L, Devries S, Chin K, Scott GK, Benz CC, Chen YY, Waldman FM, Hwang ES (2013) FOXP3-positive regulatory T lymphocytes and epithelial FOXP3 expression in synchronous normal, ductal carcinoma in situ, and invasive cancer of the breast. Breast Cancer Res Treat 139:381–390
Ortiz-Martinez F, Gutierrez-Avino FJ, Sanmartin E, Pomares-Navarro E, Villalba-Riquelme C, Garcia-Martinez A, Lerma E, Peiro G (2016) Association of Notch pathway down-regulation with Triple Negative/Basal-like breast carcinomas and high tumor-infiltrating FOXP3+ Tregs. Exp Mol Pathol 100:460–468
Droeser RA, Obermann EC, Wolf AM, Wallner S, Wolf D, Tzankov A (2013) Negligible nuclear FOXP3 expression in breast cancer epithelial cells compared with FOXP3-positive T cells. Clin Breast Cancer 13:264–270
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26:259–271
Acknowledgements
We thank Cristina Pomares and Laura Andreu for their technical assistance and Dr. Hwai-Shi Wang for his experimental design advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Institutional Ethics Committees approved the protocol.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no competing interests.
Funding
This study was supported by grants from the Instituto de Salud Carlos III (FIS PI10/00082), Conselleria de Sanitat-Generalitat Valenciana (AP-172/10), and Fundación de la Comunidad Valenciana para la Investigación, FCVI-HGUA (2011/PC-03; UGP-14-271).
Additional information
Elena Sanmartín and Fernando Ortiz-Martínez contributed equally to this article.
Rights and permissions
About this article
Cite this article
Sanmartín, E., Ortiz-Martínez, F., Pomares-Navarro, E. et al. CD44 induces FOXP3 expression and is related with favorable outcome in breast carcinoma. Virchows Arch 470, 81–90 (2017). https://doi.org/10.1007/s00428-016-2045-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-2045-3