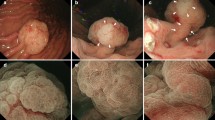
Abstract
Frequent activation of the Wnt/β-catenin signaling pathway has recently been demonstrated in gastric adenocarcinoma/neoplasia of chief cell predominant type (GA-CCP/GN-CCP) with submucosal involvement. In this study, we examined the activation status of the Wnt/β-catenin signaling pathway in GN-CCP without submucosal involvement, which is referred to as gastric dysplasia-CCP (GD-CCP). We also examined β-catenin expression and the mutation spectrum of PPP2R1A and Wnt pathway genes in 11 cases of GD-CCP, 25 cases of gastric polyps of fundic gland type (GPs-FG), and 21 cases of GPs-FG with dysplasia (GP-FGD). β-catenin nuclear staining was observed in 3 cases of GD-CCP, none of GPs-FG, and 6 cases of GPs-FGD. Mutations in Wnt pathway genes, including PPP2R1A, were observed in 4 cases of GDs-CCP, 10 cases of GPs-FG, and 7 cases of GPs-FGD. Two of these seven GPs-FGD cases showed β-catenin nuclear staining. However, none of the 4 GD-CCP cases with mutations or the 10 GPs-FG cases with mutations showed β-catenin nuclear staining. PPP2R1A mutations were observed in 1 GD-CCP case and 1 GPs-FGD case. Although the mutation spectra of the Wnt pathway genes in GD-CCP and GP-FG differed, based on the absence of β-catenin nuclear staining despite the genetic alterations, GD-CCP is more similar to GP-FG than to GN-CCP, which shows β-catenin nuclear staining and submucosal involvement. Activation of the Wnt/β-catenin signaling by the β-catenin nuclear transition may be required during progression from GD-CCP to GN-CCP. Furthermore, this is the first report describing PPP2R1A mutations in gastric fundic gland-associated neoplasms.




Similar content being viewed by others
References
Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S (2010) Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol 34:609–619. doi:10.1097/PAS.0b013e3181d94d53
Hidaka Y, Mitomi H, Saito T, Takahashi M, Lee SY, Matsumoto K, Yao T, Watanabe S (2013) Alteration in the Wnt/beta-catenin signaling pathway in gastric neoplasias of fundic gland (chief cell predominant) type. Hum Pathol 44:2438–2448. doi:10.1016/j.humpath.2013.06.002
Chen WC, Rodriguez-Waitkus PM, Barroso A, Balsaver A, McKechnie JC (2012) A rare case of gastric fundic gland adenocarcinoma (chief cell predominant type). J Gastrointest Cancer 43:262–265. doi:10.1007/s12029-012-9416-z
Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S (2014) Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy 46:153–157. doi:10.1055/s-0033-1359042
Fukatsu H, Miyoshi H, Ishiki K, Tamura M, Yao T (2011) Gastric adenocarcinoma of fundic gland type (chief cell predominant type) treated with endoscopic aspiration mucosectomy. Dig Endosc 23:244–246. doi:10.1111/j.1443-1661.2011.01125.x
Singhi AD, Lazenby AJ, Montgomery EA (2012) Gastric adenocarcinoma with chief cell differentiation: a proposal for reclassification as oxyntic gland polyp/adenoma. Am J Surg Pathol 36:1030–1035. doi:10.1097/PAS.0b013e31825033e7
Burt RW (2003) Gastric fundic gland polyps. Gastroenterology 125:1462–1469
Turner JR, Odze RD (2009) Polyps of the stomach. In: Odze RD, Goldblum JR (eds) Surgical pathology of the GI tract, liver, biliary tract, and pancreas. Saunders Elsevier, Philadelphia, pp 415–445
Wu TT, Kornacki S, Rashid A, Yardley JH, Hamilton SR (1998) Dysplasia and dysregulation of proliferation in foveolar and surface epithelia of fundic gland polyps from patients with familial adenomatous polyposis. Am J Surg Pathol 22:293–298
Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT (2000) Fundic gland polyps in familial adenomatous polyposis: neoplasms with frequent somatic adenomatous polyposis coli gene alterations. Am J Pathol 157:747–754. doi:10.1016/S0002-9440(10)64588-9
Sekine S, Shimoda T, Nimura S, Nakanishi Y, Akasu T, Katai H, Gotoda T, Shibata T, Sakamoto M, Hirohashi S (2004) High-grade dysplasia associated with fundic gland polyposis in a familial adenomatous polyposis patient, with special reference to APC mutation profiles. Mod Pathol 17:1421–1426. doi:10.1038/modpathol.3800178
Abraham SC, Park SJ, Mugartegui L, Hamilton SR, Wu TT (2002) Sporadic fundic gland polyps with epithelial dysplasia: evidence for preferential targeting for mutations in the adenomatous polyposis coli gene. Am J Pathol 161:1735–1742. doi:10.1016/S0002-9440(10)64450-1
Jalving M, Koornstra JJ, Boersma-van Ek W, de Jong S, Karrenbeld A, Hollema H, de Vries EG, Kleibeuker JH (2003) Dysplasia in fundic gland polyps is associated with nuclear beta-catenin expression and relatively high cell turnover rates. Scand J Gastroenterol 38:916–922
Cho KH, Baek S, Sung MH (2006) Wnt pathway mutations selected by optimal beta-catenin signaling for tumorigenesis. FEBS Lett 580:3665–3670. doi:10.1016/j.febslet.2006.05.053
He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H (1997) A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science 275:1652–1654
Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT (1996) A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol 6:1302–1306
Smalley MJ, Dale TC (1999) Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev 18:215–230
Kishida M, Koyama S, Kishida S, Matsubara K, Nakashima S, Higano K, Takada R, Takada S, Kikuchi A (1999) Axin prevents Wnt-3a-induced accumulation of beta-catenin. Oncogene 18:979–985. doi:10.1038/sj.onc.1202388
Li L, Yuan H, Weaver CD, Mao J, Farr GH 3rd, Sussman DJ, Jonkers J, Kimelman D, Wu D (1999) Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J 18:4233–4240. doi:10.1093/emboj/18.15.4233
Itoh K, Antipova A, Ratcliffe MJ, Sokol S (2000) Interaction of dishevelled and Xenopus axin-related protein is required for wnt signal transduction. Mol Cell Biol 20:2228–2238
Cadigan KM, Nusse R (1997) Wnt signaling: a common theme in animal development. Genes Dev 11:3286–3305
Nakamura T, Hamada F, Ishidate T, Anai K, Kawahara K, Toyoshima K, Akiyama T (1998) Axin, an inhibitor of the Wnt signalling pathway, interacts with beta-catenin, GSK-3beta and APC and reduces the beta-catenin level. Genes Cell 3:395–403
Hsu W, Zeng L, Costantini F (1999) Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem 274:3439–3445
Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A (1998) Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J 17:1371–1384. doi:10.1093/emboj/17.5.1371
Itoh K, Krupnik VE, Sokol SY (1998) Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr Biol 8:591–594
Ratcliffe MJ, Itoh K, Sokol SY (2000) A positive role for the PP2A catalytic subunit in Wnt signal transduction. J Biol Chem 275:35680–35683. doi:10.1074/jbc.C000639200
Ogasawara N, Tsukamoto T, Mizoshita T, Inada K, Cao X, Takenaka Y, Joh T, Tatematsu M (2006) Mutations and nuclear accumulation of beta-catenin correlate with intestinal phenotypic expression in human gastric cancer. Histopathology 49:612–621. doi:10.1111/j.1365-2559.2006.02560.x
Woo DK, Kim HS, Lee HS, Kang YH, Yang HK, Kim WH (2001) Altered expression and mutation of beta-catenin gene in gastric carcinomas and cell lines. Int J Cancer 95:108–113
Sasaki Y, Morimoto I, Kusano M, Hosokawa M, Itoh F, Yanagihara K, Imai K, Tokino T (2001) Mutational analysis of the beta-catenin gene in gastric carcinomas. Tumour Biol 22:123–130
Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, Groden J, Lowy AM (2002) Beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res 62:3503–3506
Yagi OK, Akiyama Y, Ohkura Y, Ban S, Endo M, Saitoh K, Yuasa Y (1997) Analyses of the APC and TGF-beta type II receptor genes, and microsatellite instability in mucosal colorectal carcinomas. Jpn J Cancer Res 88:718–724
Lagarde A, Rouleau E, Ferrari A, Noguchi T, Qiu J, Briaux A, Bourdon V, Remy V, Gaildrat P, Adelaide J, Birnbaum D, Lidereau R, Sobol H, Olschwang S (2010) Germline APC mutation spectrum derived from 863 genomic variations identified through a 15-year medical genetics service to French patients with FAP. J Med Genet 47:721–722. doi:10.1136/jmg.2010.078964
Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT (2001) Sporadic fundic gland polyps: common gastric polyps arising through activating mutations in the beta-catenin gene. Am J Pathol 158:1005–1010
Sekine S, Shibata T, Yamauchi Y, Nakanishi Y, Shimoda T, Sakamoto M, Hirohashi S (2002) Beta-catenin mutations in sporadic fundic gland polyps. Virchows Arch 440:381–386. doi:10.1007/s004280100527
Nomura R, Saito T, Mitomi H, Hidaka Y, Lee SY, Watanabe S, Yao T (2014) GNAS mutation as an alternative mechanism of activation of the Wnt/beta-catenin signaling pathway in gastric adenocarcinoma of the fundic gland type. Hum Pathol 45:2488–2496. doi:10.1016/j.humpath.2014.08.016
Hassan A, Yerian LM, Kuan SF, Xiao SY, Hart J, Wang HL (2004) Immunohistochemical evaluation of adenomatous polyposis coli, beta-catenin, c-Myc, cyclin D1, p53, and retinoblastoma protein expression in syndromic and sporadic fundic gland polyps. Hum Pathol 35:328–334
Jones S, Wang TL, Shih IM, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA, Vogelstein B, Kinzler KW, Velculescu VE, Papadopoulos N (2010) Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 330:228–231. doi:10.1126/science.1196333
Shih IM, Panuganti PK, Kuo KT, Mao TL, Kuhn E, Jones S, Velculescu VE, Kurman RJ, Wang TL (2011) Somatic mutations of PPP2R1A in ovarian and uterine carcinomas. Am J Pathol 178:1442–1447. doi:10.1016/j.ajpath.2011.01.009
Rahman M, Nakayama K, Rahman MT, Nakayama N, Katagiri H, Katagiri A, Ishibashi T, Ishikawa M, Iida K, Otsuki Y, Nakayama S, Miyazaki K (2013) PPP2R1A mutation is a rare event in ovarian carcinoma across histological subtypes. Anticancer Res 33:113–118
Janssens V, Goris J (2001) Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 353(Pt 3):417–439
Janssens V, Goris J, Van Hoof C (2005) PP2A: the expected tumor suppressor. Curr Opin Genet Dev 15:34–41. doi:10.1016/j.gde.2004.12.004
Mumby M (2007) PP2A: unveiling a reluctant tumor suppressor. Cell 130:21–24. doi:10.1016/j.cell.2007.06.034
Bos CL, Kodach LL, van den Brink GR, Diks SH, van Santen MM, Richel DJ, Peppelenbosch MP, Hardwick JC (2006) Effect of aspirin on the Wnt/beta-catenin pathway is mediated via protein phosphatase 2A. Oncogene 25:6447–6456. doi:10.1038/sj.onc.1209658
Wu MY, Xie X, Xu ZK, Xie L, Chen Z, Shou LM, Gong FR, Xie YF, Li W, Tao M (2014) PP2A inhibitors suppress migration and growth of PANC-1 pancreatic cancer cells through inhibition on the Wnt/β-catenin pathway by phosphorylation and degradation of β-catenin. Oncol Rep 32:513–522. doi:10.3892/or.2014.3266
Acknowledgments
The authors thank Dr. Minako Hirahashi (Department of Anatomic Pathology, Pathological Sciences, Graduate School of Medical Sciences, Kyushu University), Dr. Yumi Oshiro (Department of Pathology, Matsuyama Red Cross Hospital), Dr. Takehiro Tanaka (Department of Pathology, Okayama University Graduate School of Medicine), Dr. Yutaka Nakashima (Division of Pathology, Japanese Red Cross Fukuoka Hospital), Dr. Tetsumi Yamane (Department of Pathology, Tottori Red Cross Hospital), Dr. Fumiyoshi Fushimi (Department of Pathology, National Kyushu Cancer Center), Dr. Shinji Kono (Division of Clinical Pathology, Harasanshin Hospital), Dr. Shuichi Ohara (Department of Gastroenterology, Tohoku Rosai Hospital), Dr. Koyu Suzuki (Department of Pathology, St Luke’s International Hospital), and Dr. Takeshi Yano (Department of Surgery, Asoka Hospital) for kindly providing samples and clinical information, Dr. Ayumi Osako (Department of Gastroenterology, Tottori Seikyo Hospital). We also wish to thank Mrs. Keiko Mitani (Department of Human Pathology, Juntendo University School of Medicine) for her expert technical assistance. We also thank the Laboratory of Molecular and Biochemical Research, Research Support Center, Juntendo University Graduate School of Medicine, Tokyo, Japan, for technical assistance.
Funding
This work was supported, in part, by a Grant-in-Aid for General Scientific Research from the Ministry of Education, Science, Sports, and Culture (#26670286 to Tsuyoshi Saito, #24590429 to Hiroyuki Mitomi and #26460428 to Takashi Yao), Tokyo, Japan.
Conflict of interest
The authors declare that there are no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, SY., Saito, T., Mitomi, H. et al. Mutation spectrum in the Wnt/β-catenin signaling pathway in gastric fundic gland-associated neoplasms/polyps. Virchows Arch 467, 27–38 (2015). https://doi.org/10.1007/s00428-015-1753-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1753-4