Abstract
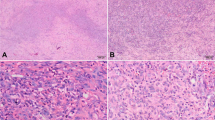
Epithelioid hemangioendothelioma is a rare vascular tumor of borderline malignancy characterized by recurrent WWTR1-CAMTA1 gene fusions in approximately 90 % of cases. In addition, a recurrent YAP1-TFE3 gene fusion has been identified in WWTR1-CAMTA1 negative epithelioid hemangioendotheliomas. This subset has been reported as having a distinct morphology with more obvious vasoformation, voluminous eosinophilic cytoplasm, and TFE3 positivity on immunohistochemistry. We report a case of a YAP1-TFE3 translocated epithelioid hemangioendothelioma arising in a groin lymph node in a 29-year-old male. Plump spindle cell morphology and absence of vasoformation made correct diagnosis particularly difficult. Immunohistochemistry showed nuclear positivity for both ERG and TFE3, fluorescence in situ hybridization showed break apart for TFE3 and RT-PCR identified a YAP1 exon1 to TFE3 exon 6 transcript, a previously unreported fusion variant. Awareness of this solid morphology and variant fusion will aid in identification of future cases of this rare vascular tumor.


Similar content being viewed by others
References
Antonescu CR (2014) Malignant vascular tumors—an update. Mod Pathol 27(Suppl 1):S30–S38
Antonescu CR, Le Loarer F, Mosquera J-M, Sboner A, Zhang L, Chen C-L, Chen H-W, Pathan N, Krausz T, Dickson BC, Weinreb I, Rubin MA, Hameed M, Fletcher CDM (2013) Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Gene Chromosome Cancer 52:775–784
Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, Healey JH, Antonescu CR (2011) A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosom Cancer 50:644–653
Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, Hanwright PJ, Flanagan J, Luo Y, Fenwick K, Natrajan R, Mitsopoulos C, Zvelebil M, Hoch BL, Weiss SW, Debiec-Rychter M, Sciot R, West RB, Lazar AJ, Ashworth A, Reis-Filho JS, Lord CJ, Gerstein MB, Rubin MA, Rubin BP (2011) Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med 3:98ra82
Flucke U, Vogels RJ, de Saint Aubain Somerhausen N, Creytens DH, Riedl RG, van Gorp JM, Milne AN, Huysentruyt CJ, Verdijk MA, van Asseldonk MM, Suurmeijer AJ, Bras J, Palmedo G, Groenen PJ, Mentzel T (2014) Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol 9:131
Williams A, Bartle G, Sumathi VP, Meis JM, Mangham DC, Grimer RJ, Kindblom L-G (2011) Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: useful diagnostic tools in cases with unusual histological features. Virchows Arch 458:291–300
Varelas X (2014) The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141:1614–1626
Vassilev A (2001) TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev 15:1229–1241
Beckmann H, Kadesch T (1991) The leucine zipper of TFE3 dictates helix-loop-helix dimerization specificity. Genes Dev 5:1057–1066
Beckmann H, Su LK, Kadesch T (1990) TFE3: a helix-loop-helix protein that activates transcription through the immunoglobulin enhancer μE3 motif. Genes Dev 4:167–179
Ladanyi M, Lui MY, Antonescu CR, Krause-Boehm A, Meindl A, Argani P, Healey JH, Ueda T, Yoshikawa H, Meloni-Ehrig A, Sorensen PH, Mertens F, Mandahl N, van den Berghe H, Sciot R, Dal Cin P, Bridge J (2001) The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 20:48–57
Weterman MA, Wilbrink M, Geurts van Kessel A (1996) Fusion of the transcription factor TFE3 gene to a novel gene, PRCC, in t(X;1)(p11;q21)-positive papillary renal cell carcinomas. Proc Natl Acad Sci U S A 93:15294–15298
Sidhar SK, Clark J, Gill S, Hamoudi R, Crew AJ, Gwilliam R, Ross M, Linehan WM, Birdsall S, Shipley J, Cooper CS (1996) The t(X;1)(p11.2;q21.2) translocation in papillary renal cell carcinoma fuses a novel gene PRCC to the TFE3 transcription factor gene. Hum Mol Genet 5:1333–1338
Argani P, Antonescu CR, Illei PB, Lui MY, Timmons CF, Newbury R, Reuter VE, Garvin AJ, Perez-Atayde AR, Fletcher JA, Beckwith JB, Bridge JA, Ladanyi M (2001) Primary renal neoplasms with the ASPL-TFE3 gene fusion of alveolar soft part sarcoma: a distinctive tumor entity previously included among renal cell carcinomas of children and adolescents. Am J Pathol 159:179–192
Argani P, Antonescu CR, Couturier J, Fournet JC, Sciot R, Debiec-Rychter M, Hutchinson B, Reuter VE, Boccon-Gibod L, Timmons C, Hafez N, Ladanyi M (2002) PRCC-TFE3 renal carcinomas: Morphologic, immunohistochemical, ultrastructural, and molecular analysis of an entity associated with the t(X;1)(p11.2;q21). Am J Surg Pathol 26:1553–1566
Clark J, Lu YJ, Sidhar SK, Parker C, Gill S, Smedley D, Hamoudi R, Linehan WM, Shipley J, Cooper CS (1997) Fusion of splicing factor genes PSF and NonO (p54nrb) to the TFE3 gene in papillary renal cell carcinoma. Oncogene 15:2233–2239
Argani P, Lui MY, Couturier J, Bouvier R, Fournet JC, Ladanyi M (2003) A novel CLTC-TFE3 gene fusion in pediatric renal adenocarcinoma with t(X;17)(p11.2;q23). Oncogene 22:5374–5378
Tanaka M, Kato K, Gomi K, Matsumoto M, Kudo H, Shinkai M, Ohama Y, Kigasawa H, Tanaka Y (2009) Perivascular epithelioid cell tumor with SFPQ/PSF-TFE3 gene fusion in a patient with advanced neuroblastoma. Am J Surg Pathol 33:1416–1420
Weterman MJ, van Groningen JJ, Jansen A, van Kessel AG (2000) Nuclear localization and transactivating capacities of the papillary renal cell carcinoma-associated TFE3 and PRCC (fusion) proteins. Oncogene 19:69–74
Kobos R, Nagai M, Tsuda M, Merl MY, Saito T, Laé M, Mo Q, Olshen A, Lianoglou S, Leslie C, Ostrovnaya I, Antczak C, Djaballah H, Ladanyi M (2013) Combining integrated genomics and functional genomics to dissect the biology of a cancer-associated, aberrant transcription factor, the ASPSCR1-TFE3 fusion oncoprotein. J Pathol 229:743–754
Tsuda M, Davis IJ, Argani P, Shukla N, McGill GG, Nagai M, Saito T, Lae M, Fisher DE, Ladanyi M (2007) TFE3 fusions activate MET signaling by transcriptional up-regulation, defining another class of tumors as candidates for therapeutic MET inhibition. Cancer Res 67:919–929
Artandi SE, Merrell K, Avitahl N, Wong K-K, Calame K (1995) TFE3 contains two activation domains, one acidic and the other proline-rich, that synergistically activate transcription. Nucleic Acids Res 23:3865–3871
Carter JM, Sukov WR, Montgomery E, Goldblum JR, Billings SD, Fritchie KJ, Folpe AL (2014) TGFBR3 and MGEA5 rearrangements in pleomorphic hyalinizing angiectatic tumors and the spectrum of related neoplasms. Am J Surg Pathol 38:1182–1992
Nayler SJ, Rubin BP, Calonje E, Chan JK, Fletcher CD (2000) Composite hemangioendothelioma: a complex, low-grade vascular lesion mimicking angiosarcoma. Am J Surg Pathol 24:352–361
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puls, F., Niblett, A., Clarke, J. et al. YAP1-TFE3 epithelioid hemangioendothelioma: a case without vasoformation and a new transcript variant. Virchows Arch 466, 473–478 (2015). https://doi.org/10.1007/s00428-015-1730-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1730-y