Abstract
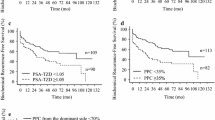
Currently, no consensus exists on the best method for tumor quantification in prostate cancer (PCA), and its prognostic value remains controversial. We evaluated how a newly defined maximum tumor diameter (MTD) might contribute to the prediction of biochemical recurrence (BCR) in a consecutive series of PCA patients treated with radical prostatectomy (RP). Patients with PCA who underwent RP without neoadjuvant therapy at a single center were included for analysis. MTD was defined as the largest diameter of all identified tumors in all three dimensions (i.e., length, width, or depth) of the prostate (“Basel technique”). Cox regression models addressed the association of MTD with BCR in three risk groups (low risk—prostate-specific antigen (PSA) < 10 ng/ml, pT2, and Gleason score (GS) ≤ 6; intermediate risk—PSA ≥ 10 and <20 ng/ml and/or pT2 and GS = 7; high risk—PSA > 20 ng/ml or pT3 or GS ≥ 8) and whole cohort. Within a median follow-up of 44 months (interquartile range (IQR) 23–66), 48 patients (9.4 %) in the intermediate-risk and high-risk groups experienced BCR. In multivariate Cox regression analysis, PSA, pathological stage (pT stage), GS, positive surgical margins (PSMs), and MTD > 19.5 mm were independent predictors for BCR (p < 0.05). In subgroup analysis, MTD as a nominal variable (<24.5 and >24.5 mm) was the only independent predictor of BCR in the intermediate-risk group (hazard ratio (HR) 9.933, 95 % confidence interval (CI) 2.070–47.665; p < 0.05). MTD is an independent risk factor of BCR in PC patients after RP. The combination of the MTD with other well-known prognostic factors after RP may improve decision-making concerning follow-up intensity or adjuvant treatment.


Similar content being viewed by others
References
van der Kwast TH, Amin MB, Billis A, Epstein JI, Griffiths D, Humphrey PA, Montironi R, Wheeler TM, Srigley JR, Egevad L, Delahunt B, IPC Group (2011) International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 2: T2 substaging and prostate cancer volume. Mod Pathol 24(1):16–25
Mizuno R, Nakashima J, Mukai M, Ookita H, Nakagawa K, Oya M, Ohigashi T, Marumo K, Murai M (2006) Maximum tumor diameter is a simple and valuable index associated with the local extent of disease in clinically localized prostate cancer. Int J Urol 13(7):951–955
Fukuhara H, Kume H, Suzuki M, Fujimura T, Enomoto Y, Nishimatsu H, Ishikawa A, Homma Y (2010) Maximum tumor diameter: a simple independent predictor for biochemical recurrence after radical prostatectomy. Prostate Cancer Prostatic Dis 13(3):244–247
Renshaw AA, Richie JP, Loughlin KR, Jiroutek M, Chung A, D’Amico AV (1999) Maximum diameter of prostatic carcinoma is a simple, inexpensive, and independent predictor of prostate-specific antigen failure in radical prostatectomy specimens. Validation in a cohort of 434 patients. Am J Clin Pathol 111(5):641–644
Nelson BA, Shappell SB, Chang SS, Wells N, Farnham SB, Smith JA Jr, Cookson MS (2006) Tumour volume is an independent predictor of prostate-specific antigen recurrence in patients undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int 97(6):1169–1172
Carvalhal GF, Humphrey PA, Thorson P, Yan Y, Ramos CG, Catalona WJ (2000) Visual estimate of the percentage of carcinoma is an independent predictor of prostate carcinoma recurrence after radical prostatectomy. Cancer 89(6):1308–1314
Fine SW, Amin MB, Berney DM, Bjartell A, Egevad L, Epstein JI, Humphrey PA, Magi-Galluzzi C, Montironi R, Stief C (2012) A contemporary update on pathology reporting for prostate cancer: biopsy and radical prostatectomy specimens. Eur Urol 62(1):20–39
Renshaw AA, Richie JP, Loughlin KR, Jiroutek M, Chung A, D’Amico AV (1998) The greatest dimension of prostate carcinoma is a simple, inexpensive predictor of prostate specific antigen failure in radical prostatectomy specimens. Cancer 83(4):748–752
Eichelberger LE, Koch MO, Eble JN, Ulbright TM, Juliar BE, Cheng L (2005) Maximum tumor diameter is an independent predictor of prostate-specific antigen recurrence in prostate cancer. Mod Pathol 18(7):886–890
Chun FK, Briganti A, Jeldres C, Gallina A, Erbersdobler A, Schlomm T, Walz J, Eichelberg C, Salomon G, Haese A, Currlin E, Ahyai SA, Benard F, Huland H, Graefen M, Karakiewicz PI (2007) Tumour volume and high grade tumour volume are the best predictors of pathologic stage and biochemical recurrence after radical prostatectomy. Eur J Cancer 43(3):536–543
Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, de Reijke TM, Verbaeys A, Bosset JF, van Velthoven R, Colombel M, van de Beek C, Verhagen P, van den Bergh A, Sternberg C, Gasser T, van Tienhoven G, Scalliet P, Haustermans K, Collette L, European Organisation for R, Treatment of Cancer RO, Genito-Urinary G (2012) Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 380(9858):2018–2027
Suardi N, Gallina A, Lista G, Gandaglia G, Abdollah F, Capitanio U, Dell’oglio P, Nini A, Salonia A, Montorsi F, Briganti A (2014) Impact of adjuvant radiation therapy on urinary continence recovery after radical prostatectomy. Eur Urol 65(3):546–551
Egevad L, Algaba F, Berney DM, Boccon-Gibod L, Griffiths DF, Lopez-Beltran A, Mikuz G, Varma M, Montironi R, European Network of U (2008) Handling and reporting of radical prostatectomy specimens in Europe: a web-based survey by the European Network of Uropathology (ENUP). Histopathology 53(3):333–339
Samaratunga H, Montironi R, True L, Epstein JI, Griffiths DF, Humphrey PA, van der Kwast T, Wheeler TM, Srigley JR, Delahunt B, Egevad L, IPC Group (2011) International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 1: specimen handling. Mod Pathol 24(1):6–15
Wise AM, Stamey TA, McNeal JE, Clayton JL (2002) Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology 60(2):264–269
Andreoiu M, Cheng L (2010) Multifocal prostate cancer: biologic, prognostic, and therapeutic implications. Hum Pathol 41(6):781–793
Dvorak T, Chen MH, Renshaw AA, Loffredo M, Richie JP, D’Amico AV (2005) Maximal tumor diameter and the risk of PSA failure in men with specimen-confined prostate cancer. Urology 66(5):1024–1028
van Oort IM, Witjes JA, Kok DE, Kiemeney LA, Hulsbergen-vandeKaa CA (2008) Maximum tumor diameter is not an independent prognostic factor in high-risk localized prostate cancer. World J Urol 26(3):237–241
D’Amico AV, Whittington R, Malkowicz SB, Cote K, Loffredo M, Schultz D, Chen MH, Tomaszewski JE, Renshaw AA, Wein A, Richie JP (2002) Biochemical outcome after radical prostatectomy or external beam radiation therapy for patients with clinically localized prostate carcinoma in the prostate specific antigen era. Cancer 95(2):281–286
Epstein JI, Carmichael M, Partin AW, Walsh PC (1993) Is tumor volume an independent predictor of progression following radical prostatectomy? A multivariate analysis of 185 clinical stage B adenocarcinomas of the prostate with 5 years of followup. J Urol 149(6):1478–1481
Ohori M, Wheeler TM, Kattan MW, Goto Y, Scardino PT (1995) Prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol 154(5):1818–1824
Salomon L, Levrel O, Anastasiadis AG, Irani J, De La Taille A, Saint F, Vordos D, Cicco A, Hoznek A, Chopin D, Abbou CC (2003) Prognostic significance of tumor volume after radical prostatectomy: a multivariate analysis of pathological prognostic factors. Eur Urol 43(1):39–44
Gervasi LA, Mata J, Easley JD, Wilbanks JH, Seale-Hawkins C, Carlton CE Jr, Scardino PT (1989) Prognostic significance of lymph nodal metastases in prostate cancer. J Urol 142(2 Pt 1):332–336
Wolters T, Roobol MJ, van Leeuwen PJ, van den Bergh RC, Hoedemaeker RF, van Leenders GJ, Schroder FH, van der Kwast TH (2010) Should pathologists routinely report prostate tumour volume? The prognostic value of tumour volume in prostate cancer. Eur Urol 57(5):821–829
Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM (1999) Biological determinants of cancer progression in men with prostate cancer. JAMA 281(15):1395–1400
Palisaar RJ, Graefen M, Karakiewicz PI, Hammerer PG, Huland E, Haese A, Fernandez S, Erbersdobler A, Henke RP, Huland H (2002) Assessment of clinical and pathologic characteristics predisposing to disease recurrence following radical prostatectomy in men with pathologically organ-confined prostate cancer. Eur Urol 41(2):155–161
McNeal JE, Villers AA, Redwine EA, Freiha FS, Stamey TA (1990) Histologic differentiation, cancer volume, and pelvic lymph node metastasis in adenocarcinoma of the prostate. Cancer 66(6):1225–1233
Cheng L, Koch MO, Juliar BE, Daggy JK, Foster RS, Bihrle R, Gardner TA (2005) The combined percentage of Gleason patterns 4 and 5 is the best predictor of cancer progression after radical prostatectomy. J Clin Oncol 23(13):2911–2917
Song C, Seo S, Ahn H, Byun SS, Cho JS, Choi YD, Lee E, Lee HM, Lee SE, Choi HY (2012) Percent tumor volume predicts biochemical recurrence after radical prostatectomy: multi-institutional data analysis. Int J Clin Oncol 17(4):355–360
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, G., Rieken, M., Bonkat, G. et al. Maximum tumor diameter adjusted to the risk profile predicts biochemical recurrence after radical prostatectomy. Virchows Arch 465, 429–437 (2014). https://doi.org/10.1007/s00428-014-1643-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1643-1