Abstract
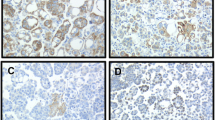
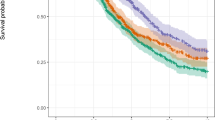
The objective of this study was to investigate the expression and clinical role of the spindle checkpoint kinase budding uninhibited by benzimidazole 1 (Bub1) in primary and metastatic advanced-stage ovarian serous carcinoma. BUB1 mRNA expression was analyzed in 178 tumors (88 effusions, 38 primary carcinomas, and 52 solid metastases) from 144 patients with advanced-stage disease using quantitative real-time polymerase chain reaction (PCR). Bub1 protein expression by Western blotting was studied in 63 carcinomas (30 effusions and 33 solid lesions). BUB1 mRNA expression at different anatomic sites was studied for association with clinicopathologic parameters, including chemotherapy resistance and survival. BUB1 mRNA was universally expressed in serous carcinomas, irrespective of anatomic site. BUB1 mRNA levels were uniformly low in six ovarian surface epithelium specimens analyzed for comparative purposes. Bub1 protein was expressed in 22/30 effusions and 28/33 solid lesions. BUB1 mRNA expression was significantly higher in chemo-naïve primary carcinomas and solid metastases compared to specimens obtained following neoadjuvant chemotherapy (p < 0.001) and was unrelated to chemotherapy exposure in effusions nor to chemoresponse or survival at any anatomic site. BUB1 mRNA levels in both effusions and solid lesions were strongly related to the mRNA levels of AURKA and AURKB previously studied in this cohort (p < 0.001 for both). Bub1 is widely expressed in primary and metastatic OC, suggesting a biological role in this cancer. BUB1 mRNA levels are lower following chemotherapy exposure in solid lesions, though its presence is unrelated to clinical behavior including response to chemotherapy and survival. BUB1 is co-expressed with AURKA and AURKB suggesting biological relationship between these spindle cell components.



Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Hennessy BT, Coleman RL, Markman M (2009) Ovarian cancer. Lancet 374:1371–1382
Malumbres M, Barbacid M (2007) Cell cycle kinases in cancer. Curr Opin Genet Dev 17:60–65
Williams GL, Roberts TM, Gjoerup OV (2007) Bub1: escapades in a cellular world. Cell Cycle 6:1699–1704
Elowe S (2011) Bub1 and BubR1: at the interface between chromosome attachment and the spindle checkpoint. Mol Cell Biol 31:3085–3093
Bolanos-Garcia VM, Blundell TL (2011) BUB1 and BUBR1: multifaceted kinases of the cell cycle. Trends Biochem Sci 36:141–150
Ricke RM, van Deursen JM (2011) Aurora B hyperactivation by Bub1 overexpression promotes chromosome missegregation. Cell Cycle 10:3645–3651
Kumar G, Breen EJ, Ranganathan S (2013) Identification of ovarian cancer associated genes using an integrated approach in a Boolean framework. BMC Syst Biol 7:12
Hetland TE, Nymoen DA, Holth A, Brusegard K, Flørenes VA, Kærn J, Tropé CG, Davidson B (2013) Aurora-B expression in metastatic effusions from advanced-stage ovarian serous carcinoma is predictive of intrinsic chemotherapy resistance. Hum Pathol 44:777–785
Elgaaen BV, Haug KB, Wang J, Olstad OK, Fortunati D, Onsrud M, Staff AC, Sauer T, Gautvik KM (2010) POLD2 and KSP37 (FGFBP2) correlate strongly with histology, stage and outcome in ovarian carcinomas. PLoS One 5:e13837
Hetland TE, Nymoen DA, Emilsen E, Kærn J, Tropé CG, Flørenes VA, Davidson B (2012) MGST1 expression in serous ovarian carcinoma differs at various anatomic sites, but is unrelated to chemoresistance or survival. Gynecol Oncol 126:460–465
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Thigpen JT, Blessing JA, Ball H, Hummel SJ, Barrett RJ (1994) Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: a Gynecologic Oncology Group study. J Clin Oncol 12:1748–1753
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Diaz-Padilla I, Oza AM (2011) Epothilones in the treatment of ovarian cancer. Future Oncol 7:559–568
Latifi A, Luwor RB, Bilandzic M, Nazaretian S, Stenvers K, Pyman J, Zhu H, Thompson EW, Quinn MA, Findlay JK, Ahmed N (2012) Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors. PLoS One 7:e46858
Vathipadiekal V, Saxena D, Mok SC, Hauschka PV, Ozbun L, Birrer MJ (2012) Identification of a potential ovarian cancer stem cell gene expression profile from advanced stage papillary serous ovarian cancer. PLoS One 7:e29079
Rizzo S, Hersey JM, Mellor P, Dai W, Santos-Silva A, Liber D, Luk L, Titley I, Carden CP, Box G, Hudson DL, Kaye SB, Brown R (2011) Ovarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2. Mol Cancer Ther 10:325–335
Meng E, Long B, Sullivan P, McClellan S, Finan MA, Reed E, Shevde L, Rocconi RP (2012) CD44+/CD24− ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin Exp Metastasis 29:939–948
Hu L, McArthur C, Jaffe RB (2010) Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br J Cancer 102:1276–1283
Lee EA, Keutmann MK, Dowling ML, Harris E, Chan G, Kao GD (2004) Inactivation of the mitotic checkpoint as a determinant of the efficacy of microtubule-targeted drugs in killing human cancer cells. Mol Cancer Ther 3:661–669
Fu Y, Ye D, Chen H, Lu W, Ye F, Xie X (2007) Weakened spindle checkpoint with reduced BubR1 expression in paclitaxel-resistant ovarian carcinoma cell line SKOV3-TR30. Gynecol Oncol 105:66–73
Lee YK, Choi E, Kim MA, Park PG, Park NH, Lee H (2009) BubR1 as a prognostic marker for recurrence-free survival rates in epithelial ovarian cancers. Br J Cancer 101:504–510
Hetland TE, Hellesylt E, Flørenes VA, Tropé C, Davidson B, Kærn J (2011) Class III β-tubulin expression in advanced-stage serous ovarian carcinoma effusions is associated with poor survival and primary chemoresistance. Hum Pathol 42:1019–1026
Kleinberg L, Flørenes VA, Silins I, Haug K, Trope’ CG, Nesland JM, Davidson B (2007) Nuclear expression of survivin is associated with improved survival in metastatic ovarian carcinoma. Cancer 109:228–238
Takagi K, Miki Y, Shibahara Y, Nakamura Y, Ebata A, Watanabe M, Ishida T, Sasano H, Suzuki T (2013) BUB1 immunolocalization in breast carcinoma: its nuclear localization as a potent prognostic factor of the patients. Horm Cancer 4:92–102
Santarpia L, Iwamoto T, Di Leo A, Hayashi N, Bottai G, Stampfer M, Andre F, Turner NC, Symmans WF, Hortobágyi GN, Pusztai L, Bianchini G (2013) DNA repair gene patterns as prognostic and predictive factors in molecular breast cancer subtypes. Oncologist 18:1063–1073
Li L, Xu DB, Zhao XL, Hao TY (2013) Combination analysis of Bub1 and Mad2 expression in endometrial cancer: act as a prognostic factor in endometrial cancer. Arch Gynecol Obstet 288:155–165
Riker AI, Enkemann SA, Fodstad O, Liu S, Ren S, Morris C, Xi Y, Howell P, Metge B, Samant RS, Shevde LA, Li W, Eschrich S, Daud A, Ju J, Matta J (2008) The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics 1:13
Swarts DR, Van Neste L, Henfling ME, Eijkenboom I, Eijk PP, van Velthuysen ML, Vink A, Volante M, Ylstra B, Van Criekinge W, van Engeland M, Ramaekers FC, Speel EJ (2013) An exploration of pathways involved in lung carcinoid progression using gene expression profiling. Carcinogenesis 34:2726–2737
Acknowledgment
This work was supported by the Inger and John Fredriksen Foundation for Ovarian Cancer Research.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Reuven Reich is affiliated with the David R. Bloom Center for Pharmacy and the Adolf and Klara Brettler Center for Research in Molecular Pharmacology and Therapeutics at The Hebrew University of Jerusalem, Israel.
Rights and permissions
About this article
Cite this article
Davidson, B., Nymoen, D.A., Elgaaen, B.V. et al. BUB1 mRNA is significantly co-expressed with AURKA and AURKB mRNA in advanced-stage ovarian serous carcinoma. Virchows Arch 464, 701–707 (2014). https://doi.org/10.1007/s00428-014-1577-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1577-7