Abstract
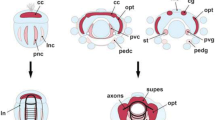
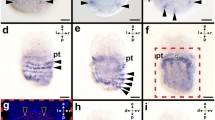
The engrailed gene is a transcription factor required in numerous species for major developmental steps (neurogenesis, limb development, boundary establishment), and its evolution is known to be closely related to the evolution of the metazoan body plan. Cephalopods exhibit numerous morphological peculiarities among molluscs, such as a direct development, a complex sensory and nervous system (eyes, brain, giant axons), a reduced shell, a funnel, and a brachial crown. We assessed a potential recruitment of engrailed in the development of these derived traits and examined the expression pattern of engrailed during the organogenesis of the cuttlefish Sepia officinalis, by immunostaining. Engrailed was detected at the margin of the prospective internal shell, which is consistent with studies on molluscs having an external shell and confirms a conserved role of engrailed in delimitating the molluscan shell compartment. Interestingly, unexpected patterns were early detected in the emerging arms, funnel and optic vesicles and latter in tentacles and eye lids. We also identified an engrailed cognate in the squid Loligo, which provides new evidence that engrailed in molluscs is not restricted to a ‘shell function’ and has been recruited in the mollusc lineage for the emergence of morphological novelties in cephalopods.





Similar content being viewed by others
References
Abzhanov A, Kaufman TC (2000) Evolution of distinct expression patterns for engrailed paralogues in higher crustaceans (Malacostraca). Dev Genes Evol 210:493–506
Boletzky S (1988) Characteristics of cephalopod embryogenesis. In: Wiedmann JKJ (ed) Cephalopods—present and past. Schweizerbartsche Verlagsbuchhandlung, Stuttgart pp 167–179
Boletzky S, Erlwein B, Hofmann DK (2006) The Sepia egg: a showcase of cephalopod embryology. Vie Milieu Life Environ 56:191–201
Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C (2005) The transcription factor Engrailed-2 guides retinal axons. Nature 438:94–98
Byrne M, Cisternas P, Elia L, Relf B (2005) Engrailed is expressed in larval development and in the radial nervous system of Patiriella sea stars. Dev Genes Evol 215:608–617
Fjose A, Mcginnis WJ, Gehring WJ (1985) Isolation of a homeobox-containing gene from the engrailed region of Drosophila and the spatial distribution of its transcripts. Nature 313:284–289
Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J (1999) Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151:1531–1545
Friedman GC, O’Leary DDM (1996) Retroviral misexpression of engrailed genes in the chick optic tectum perturbs the topographic targeting of retinal axons. J Neurosci 16:5498–5509
Gehring WJ (2005) New perspectives on eye development and the evolution of eyes and photoreceptors. J Heredity 96:171–184
Gibert J-M (2002) The evolution of engrailed genes after duplication and speciation events. Dev Genes Evol 212:307–318
Gritzan U, Hatini V, DiNardo S (1999) Mutual antagonism between signals secreted by adjacent wingless and engrailed cells leads to specification of complementary regions of the Drosophila parasegment. Development 126:4107–4115
Hidalgo A (1998) Growth and patterning from the engrailed interface. Int J Dev Biol 42:317–324
Harris WA (1997) Pax-6: where to be conserved is not conservative. Proc Natl Acad Sci 94:2421–2426
Hartmann B, Lee PN, Kang YY, Tomarev S, de Couet HG, Callaerts P (2003) Pax6 in the sepiolid squid Euprymna scolopes: evidence for a role in eye, sensory organ and brain development. Mech Dev 120:177–183
Holland L, Kene M, Williams N, Holland N (1997) Sequence and embryonic expression of the amphioxus engrailed gene (AmphiEn): the metameric pattern of transcription resembles that of its segment-polarity homolog in Drosophila. Development 124:1723–1732
Jacobs DK, Wray CG, Wedeen CJ, Kostriken R, DeSalle R, Staton JL, Gates RD, Lindberg DR (2000) Molluscan engrailed expression, serial organization and shell evolution. Evol Dev 2:340–347
Joyner AL (1996) Engrailed, Wnt and Pax genes regulate midbrain-hindbrain development. Trends Genet 12:15–20
Kornberg T (1981) Engrailed: a gene controlling compartment and segment formation in Drosophila. Proc Natl Acad Sci 78:1095–1099
Lee PN, Callaerts P, de Couet HG, Martindale MQ (2003) Cephalopod Hox genes and the origin of morphological novelties. Nature 424:1061–1965
Lemaire J (1970) Table de développement embryonnaire de Sepia officinalis L. (Mollusque Céphalopode). Bull Soc Zool 95:773–782
Lemaire J, Richard A (1978) Organogenèse de l’oeil du Céphalopode Sepia officinalis L. Bull Soc Zool Fr 103:373–377
Logan C, Wizenmann A, Drescher U, Monschau B, Bonhoeffer F, Lumsden A (1996) Rostral optic tectum acquires caudal characteristic following ectopic Engrailed expression. Curr Biol 6:1006–1014
Lowe CJ, Wray GA (1997) Radical alterations in the roles of homeobox genes during echinoderm evolution. Nature 389:718–721
Moshel SM, Levine M, Collier JR (1998) Shell differentiation and engrailed expression in the Ilyanassa embryo. Dev Genes Evol 208:135–141
Nederbragt AJ, van Loon AE, Dictus W (2002) Expression of Patella vulgata orthologs of engrailed and dpp-BMP2/4 in adjacent domains during molluscan shell development suggests a conserved compartment boundary mechanism. Dev Biol 246:341–355
Patel NH, Martin-Blanco E, Coleman KG, Poole SJ, Ellis MC, Kornberg TB, Goodman CS (1989) Expression of engrailed proteins in arthropods, annelids and chordates. Cell 58:955–968
Plaza S, Langlois M-C, Turque N, LeCornet S, Bailly M, Bègue A, Quatannens B, Dozier C, Saule S (1997) The homeobox containing Engrailed (En-1) product down-regulates the expression of Pax-6 through a DNA binding-independent mechanism. Cell Growth Differ 8:1115–1125
Quiring R, Walidorf U, Kloter U, Gehring WJ (1994) Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 265:785–789
Raftery LA, Sanicola M, Blackman RK, Gelbart WM (1991) The relationship of decapentaplegic and engrailed expression in Drosophila imaginal disks: do these genes mark the anterior–posterior compartment boundary? Development 113:27–33
Seaver EC, Kaneshige LM (2006) Expression of ‘segmentation’ genes during larval and juvenile development in the polychaetes Capitella sp. I and H. elegans. Dev Biol 289:179–194
Seaver EC, Paulson DA, Irvine SQ, Martindale MQ (2001) The spatial and temporal expression of Ch-en, the engrailed gene in the polychaete Chaetopterus, does not support a role in body segmentation. Dev Biol 236:195–209
Spiess PE (1972) Organogenese des Schalendrüsenkomplexes bei einige coleioden Cephalopoden des Mittelmeeres. Rev Suisse Zool 79:167–226
Tomarev SI, Callaerts P, Kos L, Zinovieva R, Halder G, Gehring W, Piatigorsky J (1997) Squid Pax-6 and eye development. Proc Natl Acad Sci USA 94:2421–2426
Wanninger A, Haszprunar G (2001) The expression of an engrailed protein during embryonic shell formation in the tuskshell Antalis entalis (Mollusca, Scaphopoda). Evol Dev 3:312–321
Wedeen CJ, Weisblat DA (1991) Segmental expression of an engrailed-class gene during early development and neurogenesis in an annelid. Development 113:805–814
Wray CG, Jacobs DK, Kostriken R, Vogler AP, Baker R, DeSalle R (1995) Homologues of the engrailed gene from five molluscan classes. FEBS Lett 365:71–74
Yaguchi S, Nakajima Y, Wang D, Burke RD (2006) Embryonic expression of engrailed in sea urchins. Gene Expr Patterns 6:566–571
Acknowledgments
We would like to thank S.v. Boletzky and the Observatoire Océanologique of Banyuls (Université Pierre et Marie Curie, Paris 6), L. Dickel, C. Alves and the Station Marine of Luc/Mer (Université of Caen) for providing biological material. We are grateful to M. Martin for technical help. We especially thank J. S. Deutsch and S.v. Boletzky for reading the manuscript and critical comments. The 4D9 anti-engrailed/invected antibody developed by Goodman was obtained from the Developmental studies Hybridoma Bank developed under the auspices of the NICHD and maintained at the University of Iowa, Department of Biological Sciences, Iowa City, IA52242.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D.A. Weisblat
Rights and permissions
About this article
Cite this article
Baratte, S., Andouche, A. & Bonnaud, L. Engrailed in cephalopods: a key gene related to the emergence of morphological novelties. Dev Genes Evol 217, 353–362 (2007). https://doi.org/10.1007/s00427-007-0147-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-007-0147-2