Abstract
Main conclusion
Transgenic alfalfa ( Medicago sativa L.) provides a useful reverse genetics platform to elucidate acceptor substrate specificity for uncharacterized BAHD family hydroxycinnamoyl-CoA hydroxycinnamoyl transferases.
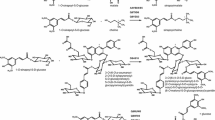
Tissues of many plant species accumulate hydroxycinnamoyl derivatives, often esters, thought to serve in protection against biotic and abiotic stresses. In many cases, these specialized metabolites are produced by BAHD family hydroxycinnamoyl-CoA hydroxycinnamoyl transferases (HCTs). Bean (Phaseolus vulgaris) leaves contain both hydroxycinnamoyl-malate esters and an HCT activity capable of making them. In seeking to identify this HCT from bean, we identified a gene whose predicted protein showed a high degree of sequence similarity (75%) to the Trifolium pratense (red clover) enzyme that carries out this reaction. The encoded bean protein, however, failed to carry out the malate transfer reaction when expressed in Escherichia coli. Expression of the gene in alfalfa (Medicago sativa) resulted in accumulation of several new hydroxycinnamates not present in nontransformed alfalfa, many of which corresponded to phenolics present in bean. Using accurate mass and UV absorption spectral data, we identified the acceptor substrate for this HCT as tetrahydroxyhexanedioic acids and demonstrated this predicted transferase activity with the E. coli-expressed protein. This finding adds to the growing number of BAHD family HCTs that have been characterized with respect to substrate specificity. Such data, combined with primary sequence and protein structural data will allow for a better understanding of the structure/function relationships of these enzymes and may eventually aid the rational design of such enzymes for altered substrate specificities. Additionally, expression of HCTs of unknown substrate specificity in alfalfa and characterization of the resulting accumulated novel metabolites could be a useful approach to characterizing putative BAHD HCT enzymes.




Similar content being viewed by others
Abbreviations
- HCT:
-
Hydroxycinnamoyl-CoA hydroxycinnamoyl transferase
- HHHT:
-
Hydroxycinnamoyl-CoA:tetrahydroxyhexandioic acid transferase
- HMT:
-
Hydroxycinnamoyl-CoA:malic acid transferase
- MS:
-
Mass spectrometry
- PDA:
-
Photodiode array
- PPO:
-
Polyphenol oxidase
References
Albrecht KA, Muck RE (1991) Proteolysis in ensiled forge legumes that vary in tannin concentration. Crop Sci 31:464–469
Bingham ET (1991) Registration of alfalfa hybrid Regen-SY germplasm for tissue culture and transformation research. Crop Sci 31:1098
Biolley JP, Kanoun M, Goulas P (2002) The response of vacuolar phenolic content of common bean (Phaseolus vulgaris cv. Bergamo) to a chronic ozone exposure: questions and hypotheses. Funct Plant Biol 29(1):1–11. doi:10.1071/Pp01163
Biolley JP, Lauga B, Cagnon C, Duran R, Salvado JC, Goulas P (1998) Phenolic pattern of bean (Phaseolus vulgaris L.) as an indicator of chronic ozone stress. Water Air Soil Poll 106(3–4):355–368. doi:10.1023/A:1005050805136
D’auria JC (2006) Acyltransferases in plants: a good time to be BAHD. Curr Opin Plant Biol 9(3):331–340
Elliger CA, Lundin RE, Haddon WF (1981) Caffeyl esters of glucaric acid in Lycopersicon esculentum leaves. Phytochemistry 20(5):1133–1134. doi:10.1016/0031-9422(81)83044-0
Eudes A, Baidoo EE, Yang F, Burd H, Hadi MZ, Collins FW, Keasling JD, Loque D (2011) Production of tranilast [N-(3’,4’-dimethoxycinnamoyl)-anthranilic acid] and its analogs in yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol 89(4):989–1000. doi:10.1007/s00253-010-2939-y
Hamid N, Bukhari N, Jawaid F (2010) Physiological responses of Phaseolus vulgaris to different lead concentrations. Pak J Bot 42(1):239–246
Harlow E, Lane D (1988) Antibodies: a laboratory manual. Cold Springs Harbor Laboratory, Cold Spring Harbor, p 508
Jones BA, Muck RE, Hatfield RD (1995) Red clover extracts inhibit legume proteolysis. J Sci Food Agric 67:329–333
Joshi CP, Zhou H, Huang XQ, Chiang VL (1997) Context sequences of translation initiation codon in plants. Plant Mol Biol 35(6):993–1001
Kuhnl T, Koch U, Heller W, Wellmann E (1987) Chlorogenic acid biosynthesis—characterization of a light-induced microsomal 5-O-(4-coumaroyl)-d-quinate/shikimate 3’-hydroxylase from carrot (Daucus carota L.) cell-suspension cultures. Arch Biochem Biophys 258(1):226–232
Lallemand LA, Zubieta C, Lee SG, Wang YC, Acajjaoui S, Timmins J, McSweeney S, Jez JM, McCarthy JG, McCarthy AA (2012) A structural basis for the biosynthesis of the major chlorogenic acids found in coffee. Plant Physiol 160(1):249–260. doi:10.1104/Pp.112.202051
Lee D, Ellard M, Wanner LA, Davis KR, Douglas CJ (1995) The Arabidopsis thaliana 4-coumarate:CoA ligase (4CL) gene: stress and developmentally regulated expression and nucleotide sequence of its cDNA. Plant Mol Biol 28(5):871–884
Lee MRF, Winters AL, Scollan ND, Dewhurst RJ, Theodorou MK, Minchin FR (2004) Plant-mediated lipolysis and proteolysis in red clover with different polyphenol oxidase activities. J Sci Food Agric 84(13):1639–1645
Lehfeldt C, Shirley AM, Meyer K, Ruegger MO, Cusumano JC, Viitanen PV, Strack D, Chapple C (2000) Cloning of the SNG1 gene of arabidopsis reveals a role for a serine carboxypeptidase-like protein as an acyltransferase in secondary metabolism. Plant Cell 12(8):1295–1306
Luo J, Nishiyama Y, Fuell C, Taguchi G, Elliott K, Hill L, Tanaka Y, Kitayama M, Yamazaki M, Bailey P, Parr A, Michael AJ, Saito K, Martin C (2007) Convergent evolution in the BAHD family of acyl transferases: identification and characterization of anthocyanin acyl transferases from Arabidopsis thaliana. Plant J 50(4):678–695. doi:10.1111/j.1365-313X.2007.03079.x
Muck RE, Dickerson JT (1988) Storage temperature effects on proteolysis in alfalfa silage. Trans ASAE 31(4):1005–1009
Nagels L, Vandongen W, Parmentier F (1982) Cestric acid, a caffeic acid ester from Cestrum euanthes. Phytochemistry 21(3):743–746. doi:10.1016/0031-9422(82)83179-8
Nielsen JK, Olsen O, Pedersen LH, Sorensen H (1984) 2-O-(p-coumaroyl)-l-malate, 2-O-caffeoyl-l-malate and 2-O-feruloyl-l-malate in Raphanus sativus. Phytochemistry 23(8):1741–1743
Niggeweg R, Michael AJ, Martin C (2004) Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat Biotechnol 22(6):746–754
Papadopoulos YA, McKersie BD (1983) A comparison of protein degradation during wilting and ensiling of six forage species. Can J Plant Sci 63:903–912
Patiny L, Borel A (2013) ChemCalc: a building block for tomorrow’s chemical infrastructure. J Chem Inf Model 53(5):1223–1228. doi:10.1021/ci300563h
Samac DA, Austin-Phillips S (2006) Alfalfa (Medicago sativa L.). In: Wang K (ed) Agrobacterium protocols. Methods in molecular biology, 2nd edn. Humana Press, Totowa, pp 301–311
Schoch GA, Morant M, Abdulrazzak N, Asnaghi C, Goepfert S, Petersen M, Ullmann P, Werck-Reichhart D (2006) The meta-hydroxylation step in the phenylpropanoid pathway: a new level of complexity in the pathway and its regulation. Environ Chem Lett 4(3):127–136
Strack D, Engel U, Weissenbock G, Grotjahn L, Wray V (1986) Ferulic acid-esters of sugar carboxylic-acids from primary leaves of rye (Secale cereale). Phytochemistry 25(11):2605–2608. doi:10.1016/S0031-9422(00)84518-5
Strack D, Keller H, Weissenbock G (1987) Enzymatic synthesis of hydroxycinnamic acid-esters of sugar acids and hydroaromatic acids by protein preparations from rye (Secale cereale) primary leaves. J Plant Physiol 131(1–2):61–73
Sullivan ML (2009a) A novel red clover hydroxycinnamoyl transferase has enzymatic activities consistent with a role in phaselic acid biosynthesis. Plant Physiol 150(4):1866–1879
Sullivan ML (2009b) Providing o-diphenols for PPO-mediated proteolytic inhibition in forage legumes: elucidating the o-diphenol biosynthetic pathway in red clover. In: Broderick GA, Adesogan AT, Bocher LW et al (eds) XVth International Silage Conference, Madison, WI, USA, pp 463–464
Sullivan ML (2014) Perennial peanut (Arachis glabrata Benth.) leaves contain hydroxycinnamoyl-CoA:tartaric acid hydroxycinnamoyl transferase activity and accumulate hydroxycinnamoyl-tartaric acid esters. Planta 239(5):1091–1100. doi:10.1007/s00425-014-2038-x
Sullivan ML, Hatfield RD (2006) Polyphenol oxidase and o-diphenols inhibit postharvest proteolysis in red clover and alfalfa. Crop Sci 46:662–670
Sullivan ML, Hatfield RD, Samac DA (2008) Cloning of an alfalfa polyphenol oxidase gene and evaluation of its potential in preventing postharvest protein degradation. J Sci Food Agric 88(8):1406–1414. doi:10.1002/Jsfa.3232
Sullivan ML, Hatfield RD, Thoma SL, Samac DA (2004) Cloning and characterization of red clover polyphenol oxidase cDNAs and expression of active protein in Escherichia coli and transgenic alfalfa. Plant Physiol 136:3234–3244
Sullivan ML, Zarnowski R (2011) Red clover HCT2, a hydroxycinnamoyl-coenzyme A: malate hydroxycinnamoyl transferase, plays a crucial role in biosynthesis of phaselic acid and other hydroxycinnamoyl-malate esters in vivo. Plant Physiol 155(3):1060–1067. doi:10.1104/Pp.110.166793
Sullivan ML, Zeller WE (2013) Efficacy of various naturally occurring caffeic acid derivatives in preventing post-harvest protein losses in forages. J Sci Food Agric 93(2):219–226. doi:10.1002/jsfa.5781
Thipyapong P, Hunt MD, Steffens JC (2004) Antisense downregulation of polyphenol oxidase results in enhanced disease susceptibility. Planta 220(1):105–117. doi:10.1007/S00425-004-1330-6
Tuominen LK, Johnson VE, Tsai CJ (2011) Differential phylogenetic expansions in BAHD acyltransferases across five angiosperm taxa and evidence of divergent expression among Populus paralogues. BMC Genom 12:236. doi:10.1186/1471-2164-12-236
Vamos-Vigyazo L (1981) Polyphenol oxidase and peroxidase in fruits and vegetables. CRC Critical Rev Food Sci Nutr 15:49–127
Verdaguer B, de Kochko A, Beachy RN, Fauquet C (1996) Isolation and expression in transgenic tobacco and rice plants, of the cassava vein mosaic virus (CVMV) promoter. Plant Mol Biol 31:1129–1139
Winters AL, Minchin FR, Michaelson-Yeates TPT, Lee MRF, Morris P (2008) Latent and active polyphenol oxidase (PPO) in red clover (Trifolium pratense) and use of a low PPO mutant to study the role of PPO in proteolysis reduction. J Agr Food Chem 56(8):2817–2824
Wise AA, Liu Z, Binns AN (2006) Three methods for the introduction of foreign DNA into Agrobacterium. In: Wang K (ed) Agrobacterium protocols, vol 1, 2nd edn. Humana Press, Totowa, pp 43–54
Acknowledgements
Thanks to Lisa Koch for excellent technical assistance and to Wayne Zeller for assistance with chemical drawings. This work was supported by the US Department of Agriculture-Agricultural Research Service and by Forage Genetics International via a collaborative research and development agreement (CRADA, 58-3K95-2-1552).
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sullivan, M.L. Identification of bean hydroxycinnamoyl-CoA:tetrahydroxyhexanedioate hydroxycinnamoyl transferase (HHHT): use of transgenic alfalfa to determine acceptor substrate specificity. Planta 245, 397–408 (2017). https://doi.org/10.1007/s00425-016-2613-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2613-4