Abstract
Main conclusion
Class II and III chitinases belonging to different glycoside hydrolase families were major nectarins in Rhododendron irroratum floral nectar which showed significant chitinolytic activity.
Previous studies have demonstrated antimicrobial activity in plant floral nectar, but the molecular basis for the mechanism is still poorly understood. Two chitinases, class II (Rhchi2) and III (Rhchi3), were characterized from alkaline Rhododendron irroratum nectar by both SDS-PAGE and mass spectrometry. Rhchi2 (27 kDa) and Rhchi3 (29 kDa) are glycoside hydrolases (family 19 and 18) with theoretical pI of 8.19 and 7.04. The expression patterns of Rhchi2 and Rhchi3 were analyzed by semi-quantitative RT-PCR. Rhchi2 is expressed in flowers (corolla nectar pouches) and leaves while Rhchi3 is expressed in flowers. Chitinase in concentrated protein and fresh nectar samples was visualised by SDS-PAGE and chitinolytic activity in fresh nectar was determined spectrophotometrically via chitin-azure. Full length gene sequences were cloned with Tail-PCR and RACE. The amino acid sequence deduced from the coding region for these proteins showed high identity with known chitinases and predicted to be located in extracellular space. Fresh R. irroratum floral nectar showed significant chitinolytic activity. Our results demonstrate that class III chitinase (GH 18 family) also exists in floral nectar. The functional relationship between class II and III chitinases and the role of these pathogenesis-related proteins in antimicrobial activity in nectar is suggested.







Similar content being viewed by others
Abbreviations
- GH:
-
Glycosyl hydrolase
References
Adrangi S, Faramarzi MA (2013) From bacteria to human: a journey into the world of chitinases. Biotechnol Adv 31:1786–1795
Akimoto C, Aoyagi H, Dicosmo F, Tanaka H (2000) Synergistic effect of active oxygen species and alginate on chitinase production by Wasabia japonica cells and its application. J Biosci Bioeng 89:131–137
Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773–784
Baker HG, Baker I (1983) A brief historical review of the chemistry of floral nectar. In: Bentley B, Elias T (eds) The biology of nectaries. Columbia University Press, New York, pp 126–152
Beintema JJ (1994) Structural features of plant chitinases and chitin-binding proteins. FEBS Lett 350:159–163
Benhamou N, Joosten MHAJ, de Wit PJGM (1990) Subcellular localization of chitinase and of potential substrate in tomato root tissue infected by Fusarium oxysporum f. sp. radicis-lycopersici. Plant Physiol 92:1108–1120
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Briesemeister S, Rahnenführer J, Kohlbacher O (2010) YLoc–an interpretable web server for predicting subcellular localization. Nucleic Acids Res 38:W497–W502
Broglie K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton S, Mauvais CJ, Broglie R (1991) Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 254:1194–1197
Brunner F, Stintzi A, Fritig B, Goddard Iii WA (1998) Substrate specificities of tobacco chitinases. Plant J 14:225–234
Carter C, Thornburg RW (2000) Tobacco nectarin I: purification and characterization as a germin-like, manganese superoxide dismutase implicated in the defense of floral reproductive tissues. J Biol Chem 275:36726–36733
Carter C, Thornburg RW (2004) Is the nectar redox cycle a floral defense against microbial attack? Trends Plant Sci 9:320–324
Chamberlain DF (1982) A revision of Rhododendron II. subgenus Hymenanthes. Notes from the Royal Botanic Garden, Edinburgh 39:209–486
Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H Jr, van Montagu M, Inzé D, van Camp W (1998) Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci USA 95:5818–5823
Chang KLB, Tai MC, Cheng FH (2001) Kinetics and products of the degradation of chitosan by hydrogen peroxide. J Agr Food Chem 49:4845–4851
Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K (1993) Plant chitinases. Plant J 3:31–40
Dore I, Legrand M, Cornelissen BJC, Bol JF (1991) Subcellular localization of acidic and basic PR proteins in tobacco mosaic virus infected tobacco. Arch Virol 120:97–107
Dubois M, Gilles KA, Hamilton JR, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Eilenberg H, Pnini-Cohen S, Schuster S, Movtchan A, Zilberstein A (2006) Isolation and characterization of chitinase genes from pitchers of the carnivorous plant Nepenthes khasiana. J Exp Bot 57:2775–2784
Emanuelsson O, Brunak S, von Heijne G, Nielsen H (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc 2:953–971
Esaka M, Teramoto T (1998) cDNA cloning, gene expression and secretion of chitinase in winged bean. Plant Cell Physiol 39:349–356
Escalante-Perez M, Jaborsky M, Reinders J, Kurzai O, Hedrich R, Ache P (2012) Poplar extrafloral nectar is protected against plant and human pathogenic fungus. Mol Plant 5:1157–1159
Evans JD, Armstrong TN (2006) Antagonistic interactions between honey bee bacterial symbionts and implications for disease. BMC Ecol 6:4. doi:10.1186/1472-6785-6-4
Ferrari M, Bjornstad O, Partain J, Antonovics J (2006) A gravity model for the spread of a pollinator-borne plant pathogen. Am Nat 168:294–303
Ferre F, Clote P (2006) DiANNA 1.1: an extension of the DiANNA web server for ternary cysteine classification. Nucleic Acids Res 34(Suppl 2): W182–W185
Flach J, Pilet PE, Jolles P (1992) What’s new in chitinase research? Experientia 48:701–716
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols Handbook. Humana Press, Totowa, pp 571–607
Gonzalez-Teuber M, Eilmus S, Muck A, Svatos A, Heil M (2009) Pathogenesis-related proteins protect extrafloral nectar from microbial infestation. Plant J 58:464–473
Gonzalez-Teuber M, Pozo MJ, Muck A, Svatos A, Adame-Alvarez RM, Heil M (2010) Glucanases and chitinases as causal agents in the protection of acacia extrafloral nectar from infestation by phytopathogens. Plant Physiol 152:1705–1715
Grover A (2012) Plant chitinases: genetic diversity and physiological roles. CRC Crit Rev Plant Sci 31:57–73
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, New York
Hamel F, Boivin R, Tremblay C, Bellemare G (1997) Structural and evolutionary relationships among chitinases of flowering plants. J Mol Evol 44:614–624
Hamid R, Khan MA, Ahmad M et al (2013) Chitinases: an update. J Pharm Bioallied Sci 5:21–29
Heil M (2011) Nectar: generation, regulation and ecological functions. Trends Plant Sci 16:191–200
Heil M (2015) Extrafloral nectar at the plant-insect interface: a spotlight on chemical ecology, phenotypic plasticity, and food webs. Annu Rev Entomol 60:213–232
Henrissat B, Davies G (1997) Structural and sequence-based classification of glycoside hydrolases. Curr Opin Struct Biol 7:637–644
Hillwig MS, Kanobe C, Thornburg RW, MacIntosh GC (2011) Identification of S-RNase and peroxidase in petunia nectar. J Plant Physiol 168:734–738
Huet J, Rucktooa P, Clantin B, Azarkan M, Looze Y, Villeret V, Wintjens R (2008) X-ray structure of papaya chitinase reveals the substrate binding mode of glycosyl hydrolase family 19 chitinases. Biochemistry 47:8283–8291
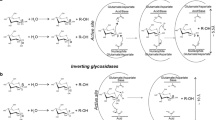
Iseli B, Armand S, Boller T, Neuhaus JM, Henrissat B (1996) Plant chitinases use two different hydrolytic mechanisms. FEBS Lett 382:186–188
Jaakola L, Pirttilä A, Halonen M, Hohtola A (2001) Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol Biotechnol 19:201–203
Jach G, Goruhardt B, Mundy J, Logemann J, Pinsdorf E, Leah R, Schell J, Maas C (1995) Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J 8:97–109
Kasprzewska A (2003) Plant chitinases-regulation and function. Cell Mol Biol Lett 8:809–824
Kikuchi T, Masuda K (2009) Class II chitinase accumulated in the bark tissue involves with the cold hardiness of shoot stems in highbush blueberry (Vaccinium corymbosum L.). Sci Hortic-Amsterdam 120:230–236
Kobayashi N, Horikoshi T, Katsuyama H, Handa T, Takayanagi K (1998) A simple and efficient DNA extraction method for plants, especially woody plants. Plant Tissue Culture Biotech 4:72–80
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Lawton K, Ward E, Payne G, Moyer M, Ryals J (1992) Acidic and basic class III chitinase mRNA accumulation in response to TMV infection of tobacco. Plant Mol Biol 19:735–743
Lin W, Hu X, Zhang W, Rogers WJ, Cai W (2005) Hydrogen peroxide mediates defence responses induced by chitosans of different molecular weights in rice. J Plant Physiol 162:937–944
Liu YG, Chen Y (2007) High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques 43:649–656
Liu D, Cai J, Xie CC, Liu C, Chen YH (2010) Purification and partial characterization of a 36-kDa chitinase from Bacillus thuringiensis subsp. colmeri, and its biocontrol potential. Enzyme Microb Tech 46:252–256
Melchers LS, Ponstein AS, Sela-Buurlage MB, Vloemans SA, Cornelissen BJC (1993) In vitro anti-microbial activities of defense proteins and biotechnology. In: Fritig B, Legrand M (eds) Mechanisms of plant defense. Kluwer Academic Publishers, Dordrecht, pp 401–410
Minic Z (2008) Physiological roles of plant glycoside hydrolases. Planta 227:723–740
Molan PC, Mizrahi A, Lensky Y (1997) Honey as an antimicrobial agent. In: Mizrahi A, Lensky Y (eds) Bee products: properties, applications and apitherapy. Plenum Press, New York, pp 27–37
Nakatsuka A, Mizuta D, Kii Y, Miyajimac I, Kobayashia N (2008) Isolation and expression analysis of flavonoid biosynthesis genes in evergreen azalea. Sci Hortic-Amsterdam 118:314–320
Neuhaus JM, Fritig B, Linthorst HJM, Meins F, Mikkelsen J, Ryals J (1996) A revised nomenclature for chitinase genes. Plant Mol Biol Rep 14:102–104
Nicolson S, Thornburg RW (2007) Nectar chemistry. In: Pacini E, Nepi M, Nicolson S (eds) Nectary and nectar: a modern treatise. Springer, Amsterdam, pp 215–263
Nocentini D, Guarnieri M, Soligo C (2015) Nectar defense and hydrogen peroxide in floral nectar of Cucurbita pepo. Acta Agrobotanica 68:187–193
Oldach KH, Becker D, Lorz H (2001) Heterologous expression of genes mediating enhanced fungal resistance in transgenic wheat. Mol Plant Microbe Interact 14:832–838
Pacini E, Nicolson SW (2007) Introduction. In: Nicolson SW, Nepi M, Pacini E (eds) Nectaries and nectar. Springer, Dordrecht, pp 1–18
Park CH, Kim S, Park JY, Ahn IP, Jwa NS, Im KH, Lee YH (2004) Molecular characterization of a pathogenesis-related protein 8 gene encoding a class III chitinase in rice. Mol Cells 17:144–150
Peng Y, Arora R, Li GW, Wang X, Fessehaie A (2008) Rhododendron catawbiense plasma membrane intrinsic proteins are aquaporins, and their over-expression compromises constitutive freezing tolerance and cold acclimation ability of transgenic Arabidopsis plants. Plant, Cell Environ 31:1275–1289
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786
Primack RB (1985) Longevity of individual flowers. Annu Rev Ecol Syst 16:15–37
Prŷs-Jones OE, Willmer PG (1992) The biology of alkaline nectar in the purple toothwort (Lathraea clandestina): ground level defences. Biol J Linn Soc 45:373–388
Schagger H, von Jagow G (1987) Tricine-sodium dodecyl sulfatepolyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Schlumbaum A, Mauch F, Vögeli U, Boller T (1986) Plant chitinases are potent inhibitors of fungal growth. Nature 324:365–367
Seo PJ, Wielsch N, Kessler D, Svatos A, Park CM, Baldwin IT, Kim SG (2013) Natural variation in floral nectar proteins of two Nicotiana attenuata accessions. BMC Plant Biol 13:101
Singh A, Isaac Kirubakaran S, Sakthivel N (2007) Heterologous expression of new antifungal chitinase from wheat. Protein Expres Purif 56:100–109
Takenaka Y, Nakano S, Tamoi M, Sakuda S, Fukamizo T (2009) Chitinase gene expression in response to environmental stresses in Arabidopsis thaliana: chitinase inhibitor allosamidin enhances stress tolerance. Biosci Biotech Biochem 73:1066–1071
Trudel J, Asselin A (1989) Detection of chitinase activity after polyacrylamide gel electrophoresis. Anal Biochem 178:362–366
Udaya Prakash NA, Jayanthi M, Sabarinathan R, Sabarinathan R, Kangueane P, Mathew L, Sekar K (2010) Evolution, homology conservation, and identification of unique sequence signatures in GH19 family chitinases. J Mol Evol 70:466–478
Vitale A, Chrispeels MJ (1992) Sorting of proteins to the vacuoles of plant cells. BioEssays 14:151–160
Wagner R, Mugnaini S, Sniezko R, Hardie D, Poulis B, Nepi M, Pacini E, von Aderkas P (2007) Proteomic evaluation of gymnosperm pollination drop proteins indicates highly conserved and complex biological functions. Sex Plant Reprod 20:181–189
Weston RJ (2000) The contribution of catalase and other natural products to the antibacterial activity of honey: a review. Food Chem 71:235–239
Zha HG, Milne RI, Sun H (2010) Asymmetric hybridization in Rhododendron agastum: a hybrid taxon comprising mainly F1 s in Yunnan, China. Ann Bot 105:89–100
Acknowledgments
We thank Prof. Bernard Henrissat (CNRS) for his suggestions and critical reading of the manuscript. This study was supported by National Science Foundation of China (Grant No. 31170216 to HG Zha) and Major Program of National Natural Science Foundation of China (Grant No. 31590823 to Hang Sun).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
Our work complies to the ethical rules applicable for this journal.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
MS spectra of identified proteins and fragments. a Rhchi2 MS spectra. b MS/MS spectra of m/z 1451.65, “GFYTYEAFI(L)AAAK” fragment in Rhchi2. c MS/MS spectra of m/z 2642.2, “TAL(I)WFWMTPQSPKPSSHDVITGR” fragment in Rhchi2. d Rhchi3 MS spectra. e MS/MS spectra of m/z 1110.5, “YGGI(L)ML(I)WDR” fragment in Rhchi3. f MS/MS spectra of m/z 1526.77, “I(L)VNL(I)GFL(I)SAFGNFK” fragment in Rhchi3 (TIFF 2255 kb)
Supplementary Fig. S2
Comparison of Rhchi2 amino acid sequence with that of six class II plant chitinase homologues. Amino acids, which are completely conserved are marked with asterisks, and the highly conserved amino acids are marked with dots or double dots. -, gap left to improve alignment. Numbers refer to amino acid residues at the end of the respective lines. Species names are abbreviated at the left and represent with accession number: Zmchi2 (Zea mays, B6SZC6), Gmchi2 (Glycine max, C6TNB0), Ntchi2 (Nicotiana tabacum, Q9ZWS3), Vvchi2 (Vitis vinifera, A5AT00), Qschi2 (Oryza sativa, Q7XCK6), Ghchi2 (Gossypium hirsutum, P931545) (DOC 105 kb)
Supplementary Fig. S3
Comparison of Rhchi3 amino acid sequence with that of six class III plant chitinase homologues. Amino acids, which are completely conserved are marked with asterisks, and the highly conserved amino acids are marked with dots or double dots. -, gap left to improve alignment. Numbers refer to amino acid residues at the end of the respective lines. Species names are abbreviated at the left and represent an accession number: Zmchi3 (Zea mays, B4G1T3), Gmchi3 (Glycine max, C6T8G2), Ntchi3 (Nicotiana tabacum, P29061), Vvchi3 (Vitis vinifera, Q84S31), Qschi3 (Oryza sativa, Q84ZH2), Ghchi3 (Gossypium hirsutum, A2TJX5) (DOC 110 kb)
Rights and permissions
About this article
Cite this article
Zha, HG., Milne, R.I., Zhou, HX. et al. Identification and cloning of class II and III chitinases from alkaline floral nectar of Rhododendron irroratum, Ericaceae. Planta 244, 805–818 (2016). https://doi.org/10.1007/s00425-016-2546-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2546-y