Abstract
Main conclusion
The total capacity of the GS-mediated ligation of free ammonium and glutamate to form glutamine in the leaves of maize plants is not impaired upon severe magnesium starvation. Magnesium deficiency does not obligatorily lead to the decreased total protein concentrations in the leaves.
Abstract
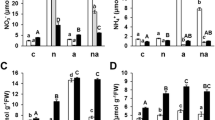
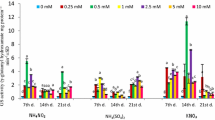
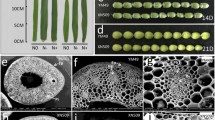
Magnesium (Mg) is an integral component of the enzyme glutamine synthetase (GS), having both a structural and a catalytic role. Moreover, Mg is relevant for the post-translational regulation of the GS. Glutamine synthetase is one of the key enzymes in nitrogen assimilation, ligating-free ammonium (NH4 +) to glutamate to form glutamine and it is therefore crucial for plant growth and productivity. This study was conducted in order to test whether a severe Mg-deficiency impairs the total capacity of the GS-catalyzed synthesis of glutamine in maize leaves. Maize was grown hydroponically and the GS activity was analyzed dependent on different leaf developmental stages. Glutamine synthetase activity in vitro assays in combination with immune-dot blot analysis revealed that both the total activity and the abundance of glutamine synthetase was not impaired in the leaves of maize plants upon 54 days of severe Mg starvation. Additionally, it was shown that Mg deficiency does not obligatorily lead to decreased total protein concentrations in the leaves, as assayed by Bradford protein quantification. Moreover, Mg resupply to the roots or the leaves of Mg-deficient plants reversed the Mg-deficiency-induced accumulation of free amino acids in older leaves, which indicates impaired phloem loading. The results of our study reveal that the total GS-mediated primary or secondary assimilation of free NH4 + is not a limiting enzymatic reaction under Mg deficiency and thus cannot be accountable for the observed restriction of plant growth and productivity in Mg-deficient maize.





Similar content being viewed by others
Abbreviations
- Asn:
-
Asparagine
- FW:
-
Fresh weight
- GHA:
-
γ-glutamyl hydroxamate
- Gln:
-
Glutamine
- Glu:
-
Glutamate
- GS:
-
Glutamine synthetase
- NS:
-
Nutrient solution
References
Balazadeh S, Schildhauer J, Araujo WL, Munne-Bosch S, Fernie AR, Proost S, Humbeck K, Mueller-Roeber B (2014) Reversal of senescence by N resupply to N-starved Arabidopsis thaliana: transcriptomic and metabolomic consequences. J Exp Bot 65:3975–3992. doi:10.1093/Jxb/Eru119
Balke NE, Hodges TK (1975) Plasma-membrane adenosine-triphosphatase of oat roots—activation and inhibition by Mg2+ and ATP. Plant Physiol 55:83–86. doi:10.1104/pp.55.1.83
Bernard SM, Habash DZ (2009) The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol 182:608–620. doi:10.1111/j.1469-8137.2009.02823.x
Betti M, Garcia-Calderon M, Perez-Delgado CM, Credali A, Estivill G, Galvan F, Vega JM, Marquez AJ (2012) Glutamine synthetase in legumes: recent advances in enzyme structure and functional genomics. Int J Mol Sci 13:7994–8024. doi:10.3390/Ijms13077994
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1006/abio.1976.9999
Cakmak I, Hengeler C, Marschner H (1994) Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J Exp Bot 45:1251–1257. doi:10.1093/jxb/45.9.1251
Caputo C, Criado MV, Roberts IN, Gelso MA, Barneix AJ (2009) Regulation of glutamine synthetase 1 and amino acids transport in the phloem of young wheat plants. Plant Physiol Biochem 47:335–342. doi:10.1016/j.plaphy.2009.01.003
Chatterjee C, Nautiyal N, Agarwala SC (1994) Influence of changes in manganese and magnesium supply on some aspects of wheat physiology. Soil Sci Plant Nutr 40:191–197
Ding Y, Luo W, Xu G (2006) Characterisation of magnesium nutrition and interaction of magnesium and potassium in rice. Ann Appl Biol 149:111–123. doi:10.1111/j.1744-7348.2006.00080.x
Eisenberg D, Gill HS, Pfluegl GMU, Rotstein SH (2000) Structure-function relationships of glutamine synthetases. Biochim Biophys Acta 1477:122–145. doi:10.1016/S0167-4838(99)00270-8
Finnemann J, Schjoerring JK (2000) Post-translational regulation of cytosolic glutamine synthetase by reversible phosphorylation and 14-3-3 protein interaction. Plant J 24:171–181. doi:10.1046/j.1365-313x.2000.00863.x
Fischer ES, Lohaus G, Heineke D, Heldt HW (1998) Magnesium deficiency results in accumulation of carbohydrates and amino acids in source and sink leaves of spinach. Physiol Plant 102:16–20. doi:10.1034/j.1399-3054.1998.1020103.x
Gallais A, Hirel B (2004) An approach to the genetics of nitrogen use efficiency in maize. J Exp Bot 55:295–306. doi:10.1093/Jxb/Erh006
Gransee A, Führs H (2013) Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 368:5–21. doi:10.1007/s11104-012-1567-y
Hanstein S, Wang XZ, Qian XQ, Friedhoff P, Fatima A, Shan YH, Feng K, Schubert S (2011) Changes in cytosolic Mg2+ levels can regulate the activity of the plasma membrane H+-ATPase in maize. Biochem J 435:93–101. doi:10.1042/Bj20101414
Hermans C, Bourgis F, Faucher M, Strasser RJ, Delrot S, Verbruggen N (2005) Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 220:541–549. doi:10.1007/s00425-004-1376-5
Hirel B, Andrieu B, Valadier MH, Renard S, Quillere I, Chelle M, Pommel B, Fournier C, Drouet JL (2005a) Physiology of maize II: identification of physiological markers representative of the nitrogen status of maize (Zea mays) leaves during grain filling. Physiol Plant 124:178–188. doi:10.1111/j.1399-3054.2005.00511.x
Hirel B, Martin A, Terce-Laforgue T, Gonzalez-Moro MB, Estavillo JM (2005b) Physiology of maize I: a comprehensive and integrated view of nitrogen metabolism in a C4 plant. Physiol Plant 124:167–177. doi:10.1111/j.1399-3054.2005.00510.x
Ireland RJ, Lea PJ (1999) The enzymes of glutamine, glutamate, asparagine, and aspartate metabolism. In: Singh B (ed) Plant amino acids: biochemistry and biotechnology. Marcel Dekker, New York, pp 49–109
Jezek M, Geilfus CM, Bayer A, Muehling KH (2015) Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front Plant Sci 5:Art 781. doi: 10.3389/Fpls.2014.00781
Kaiser WM, Weiner H, Huber SC (1999) Nitrate reductase in higher plants: a case study for transduction of environmental stimuli into control of catalytic activity. Physiol Plant 105:385–390
Kamachi K, Yamaya T, Mae T, Ojima K (1991) A role for glutamine synthetase in the remobilization of leaf nitrogen during natural senescence in rice leaves. Plant Physiol 96:411–417. doi:10.1104/pp.96.2.411
Kichey T, Le Gouis J, Sangwan B, Hirel B, Dubois F (2005) Changes in the cellular and subcellular localization of glutamine synthetase and glutamate dehydrogenase during flag leaf senescence in wheat (Triticum aestivum L.). Plant Cell Physiol 46:964–974. doi:10.1093/Pcp/Pci105
Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW (2003) Phloem loading and unloading of sugars and amino acids. Plant Cell Environ 26:37–56. doi:10.1046/j.1365-3040.2003.00847.x
Lea PJ, Sodek L, Parry MAJ, Shewry R, Halford NG (2007) Asparagine in plants. Ann Appl Biol 150:1–26. doi:10.1111/j.1744-7348.2006.00104.x
Lima L, Seabra A, Melo P, Cullimore J, Carvalho H (2006) Phosphorylation and subsequent interaction with 14-3-3 proteins regulate plastid glutamine synthetase in Medicago truncatula. Planta 223:558–567. doi:10.1007/s00425-005-0097-8
Martin A, Lee J, Kichey T, Gerentes D, Zivy M, Tatout C, Dubois F, Balliau T, Valot B, Davanture M, Terce-Laforgue T, Quillere I, Coque M, Gallais A, Gonzalez-Moro MB, Bethencourt L, Habash DZ, Lea PJ, Charcosset A, Perez P, Murigneux A, Sakakibara H, Edwards KJ, Hirel B (2006) Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. Plant Cell 18:3252–3274. doi:10.1105/tpc.106.042689
Masclaux C, Valadier MH, Brugiere N, Morot-Gaudry JF, Hirel B (2000) Characterization of the sink/source transition in tobacco (Nicotiana tabacum L.) shoots in relation to nitrogen management and leaf senescence. Planta 211:510–518. doi:10.1007/s004250000310
Mengel K, Kirkby EA (2001) Principles of plant nutrition, 5th edn./edited by Konrad Mengel and Ernest A. Kirkby with the support of Harald Kosegarten and Thomas Appel. Kluwer, Dordrecht
Okumoto S, Pilot G (2011) Amino acid export in plants: a missing link in nitrogen cycling. Mol Plant 4:453–463. doi:10.1093/Mp/Ssr003
O’Neal D, Joy KW (1973) Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch Biochem Biophys 159:113–122. doi:10.1016/0003-9861(73)90435-9
O’Neal D, Joy KW (1974) Glutamine synthetase of pea leaves —divalent cation effects, substrate specificity, and other properties. Plant Physiol 54:773–779. doi:10.1104/Pp.54.5.773
Rhodes D, Rendon GA, Stewart GR (1975) Control of glutamine synthetase level in Lemna minor L. Planta 125:201–211. doi:10.1007/Bf00385596
Riedel J, Tischner R, Mack G (2001) The chloroplastic glutamine synthetase (GS-2) of tobacco is phosphorylated and associated with 14-3-3 proteins inside the chloroplast. Planta 213:396–401. doi:10.1007/s004250000509
Seabra AR, Silva LS, Carvalho HG (2013) Novel aspects of glutamine synthetase (GS) regulation revealed by a detailed expression analysis of the entire GS gene family of Medicago truncatula under different physiological conditions. BMC Plant Biol 13:Art 137. doi: 10.1186/1471-2229-13-137
Thomsen HC, Eriksson D, Moller IS, Schjoerring JK (2014) Cytosolic glutamine synthetase: a target for improvement of crop nitrogen use efficiency? Trends Plant Sci 19:656–663. doi:10.1016/j.tplants.2014.06.002
Watanabe A, Takagi N, Hayashi H, Chino M, Watanabe A (1997) Internal Gln/Glu ratio as a potential regulatory parameter for the expression of a cytosolic glutamine synthetase gene of radish in cultured cells. Plant Cell Physiol 38:1000–1006
Waters BM (2011) Moving magnesium in plant cells. New Phytol 190:510–513. doi:10.1111/j.1469-8137.2011.03724.x
Yin ST, Ze YG, Liu C, Li N, Zhou M, Duan YM, Hong FS (2009) Cerium relieves the inhibition of nitrogen metabolism of spinach caused by magnesium deficiency. Biol Trace Elem Res 132:247–258. doi:10.1007/s12011-009-8392-z
Acknowledgments
Mareike Jezek receives a scholarship from the Heinrich-Böll-Foundation which is gratefully acknowledged. Mrs. Stephanie thor Straten and Mrs. Bärbel Biegler are kindly acknowledged for excellent technical assistance. The authors thank Privatdozent Dr. Feng Yan for giving advice in calculating glutamine synthetase activity.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jezek, M., Geilfus, CM. & Mühling, KH. Glutamine synthetase activity in leaves of Zea mays L. as influenced by magnesium status. Planta 242, 1309–1319 (2015). https://doi.org/10.1007/s00425-015-2371-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2371-8