Abstract
Main conclusion
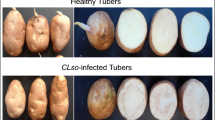
Zebra chip disease of potato decreases protease inhibitor levels resulting in enhanced serine-type protease activity, decreased protein content and altered protein profiles of fully mature tubers.
Zebra-chip (ZC), caused by Candidatus Liberibacter solanacearum (CLso), is a relatively new disease of potato that negatively affects growth, yield, propagation potential, and fresh and process qualities of tubers. Diseased plants produce tubers with characteristic brown discoloration of vascular tissue accompanied by elevated levels of free amino acids and reducing sugars. Here we demonstrate that ZC disease induces selective protein catabolism in tubers through modulating protease inhibitor levels. Soluble protein content of tubers from CLso-infected plants was 33 % lower than from non-infected plants and electrophoretic analyses revealed substantial reductions in major tuber proteins. Patatin (~40 kDa) and ser-, asp- (22 kDa) and cys-type (85 kDa) protease inhibitors were either absent or greatly reduced in ZC-afflicted tubers. In contrast to healthy (non-infected) tubers, the proteolytic activity in CLso infected tubers was high and the ability of extracts from infected tubers to inhibit trypsin (ser-type) and papain (cys-type) proteases greatly attenuated. Moreover, extracts from CLso-infected tubers rapidly catabolized proteins purified from healthy tubers (40 kDa patatin, 22 kDa protease inhibitors, 85 kDa potato multicystatin) when subjected to proteolysis individually. In contrast, crude extracts from non-infected tubers effectively inhibited the proteolytic activity from ZC-afflicted tubers. These results suggest that the altered protein profile of ZC afflicted tubers is largely due to loss of ser- and cys-type protease inhibitors. Further analysis revealed a novel PMSF-sensitive (ser) protease (ca. 80–120 kDa) in CLso infected tubers. PMSF abolished the proteolytic activities responsible for degrading patatin, the 22 kDa protease inhibitor(s) and potato multicystatin by CLso infected tubers. The disease-induced loss of patatin and protease inhibitors therefore appears to be modulated by ser-type protease(s). The selective catabolism of proteins in ZC-afflicted tubers undoubtedly affects downstream aspects of carbohydrate and amino acid metabolism, which is ultimately reflected by the accumulation of reducing sugars, free amino acids and reduced sprouting capacity.












Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- asp:
-
Aspartic
- BAPNA:
-
N α-benzoyl-l-arginine 4-nitroanilide hydrochloride
- cys:
-
Cysteine
- Pep:
-
Pepstatin
- PIN I:
-
Potato protease inhibitor I
- PIN II:
-
Potato protease inhibitor II
- PKPI:
-
Potato Kunitz-type protease inhibitor
- PMC:
-
Potato multicystatin
- ser:
-
Serine
- ZC:
-
Zebra chip
References
Alvarado VY, Odokonyero D, Duncan O, Mirov TE, Scholthof HB (2012) Molecular and physiological properties associated with zebra complex disease in potatoes and its relation with Candidatus Liberibacter contents in psyllid vectors. PLoS One 7(5):e37345. doi:10.1371/journal.pone.0037345
Andrews DL, Beames B, Summers MD, Park WD (1988) Characterization of the lipid acyl hydrolase activity of the major potato (Solanum tuberosum) tuber protein, patatin, by cloning and abundant expression in a baculovirus vector. Biochem J 252:199–206
Antão CM, Malcata FX (2005) Plant serine proteases: biochemical, physiological and molecular features. Review. Plant Physiol Biochem 43:637–650
Baek KH, Choi D (2008) Roles of plant proteases in pathogen defense. Mini-review. Plant Pathol J 24(4):367–374
Bauw G, Nielsen HV, Emmersen J, Nielsen KL, Jørgensen M, Welinder KG (2006) Patatins, Kunitz protease inhibitors and other major proteins in tuber of potato cv. Kuras. FEBS J 273:3569–3584
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buchman JL, Heilman BE, Munyaneza JE (2011) Effects of Bactericera cockerelli (Hemiptera: Triozidae) density on zebra chip potato disease incidence, potato yield, and tuber processing quality. J Econo Entomol 104:1783–1792
Casteel CL, Hansen AK, Walling LL, Paine TD (2012) Manipulation of plant defense responses by the tomato psyllid (Bactericera cockerelli) and its associated endosymbiont Candidatus Liberibacter psyllaurous. PLoS One 7(4):e35191. doi:10.1371/journal.pone.0035191
Cater SA, Lees WE, Hill J, Brzin J, Kay J, Phylip LH (2002) Aspartic proteinase inhibitors from tomato and potato are more potent against yeast proteinase A than cathepsin D. Biochim Biophys Acta 1596:76–82
Crosslin, JM, Munyaneza JE, Brown JK, Liefting LW (2010). Potato zebra-chip disease: A phytopathological tale. Online. Plant Health Prog. doi:10.1094/PHP-2010-0317-01-RV
Dreher KA, Callis J (2007) Ubiquitin, hormones and biotic stress in plants. Ann Bot 9:787–822
Gilroy EM, Hein I, van der Hoorn R, Boevink PC, Venter E, McLellan H, Kaffarnik F, Hrubikova K, Shaw J, Holeva M, López EC, Borras-Hidalgo O, Pritchard L, Loake GJ, Lacomme C, Birch PR (2007) Involvement of cathepsin B in the plant disease resistance hypersensitive response. Plant J 52:1–13
Green AR, Nissen MS, Kumar GNM, Knowles NR, Kang C (2013) Characterization of Solanum tuberosum multicystatin and the significance of core domains. Plant Cell 25:5043–5052
Greenway G (2014) Economic impact of zebra chip control costs on grower returns in seven states. Amer J Potato Res 91:714–719
Guevara MG, Oliva CR, Huartle M, Daleo GR (2002) An aspartic protease with antimicrobial activity is induced after infection and wounding in intercellular fluids of potato tubers. European J Plant Pathol 108:131–137
Guevara MG, Verissimo P, Pires E, Faro C, Daleo GR (2004) Potato aspartic proteases: induction, antimicrobial activity and substrate specificity. J Plant Path 86:233–238
Hamm PB, Rondon, SI, Crosslin JM, Munyaneza, JE (2011) A new threat in the Columbia basin of Oregon and Washington: Zebra-chip. In: Proceedings of 11th Annual SCRI Zebra-chip Reporting Session F, pp 1–5
Hannapel DJ (1993) Nucleotide and deduced amino acid sequence of the 22-kilodalton cathepsin D inhibitor protein of potato (Solanum tuberosum L.). Plant Physiol 101:703–704
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments. Plant Signal Behav 7:1456–1466
Henne DC, Workneh F, Wen A, Price JA, Pasche JS, Gudmestad NC, Rush CM (2010) Characterization and epidemiological significance of potato plants grown from seed tubers affected by zebra chip disease. Plant Dis 94:659–665
Heussen C, Dowdle EB (1980) Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem 102:196–202
Hoang JHB, Hirschberg HJH, Simons JWFA, Dekker N, Egmond MR (2001) Cloning, expression, purification and characterization of patatin, a novel phospholipase A2. Eur J Biochem 268:5037–5044
Holk A, Rietz S, Zahn M, Ouader H, Scherer GF (2002) Molecular identification of cytosolic, patatin-related phospholipases A from Arabidopsis with potential functions in plant signal transduction. Plant Physiol 130:90–101
Kim YS, Lee YH, Kim HS, Kim MS, Hahn KW, Ko JH, Joung H, Jeon JH (2008) Development of patatin knockdown potato tubers using RNA interference (RNAi) technology, for the production of human-therapeutic glycoproteins. BMC Biotechnol 8:36
Koiwa H, Bressan RA, Hasegawa PM (1997) Regulation of protease inhibitors and plant defense. Trends Plant Sci 2:379–384
Kreft S, Ravnikar M, Mesko P, Pungercar J, Umek A, Kregar I, Strukelj B (1997) Jasmonic acid inducible aspartic proteinase inhibitors from potato. J Phytochem 44:1001–1006
Kumar GNM, Houtz RL, Knowles NR (1999) Age-induced protein modifications and increased proteolysis in potato seed-tubers. Plant Physiol 119:89–100
Kumar GNM, Lulai EC, Suttle RC, Knowles NR (2010) Age-induced loss of wound-healing ability in potato tubers is partly regulated by ABA. Planta 232:1433–1445
Kumar GNM, Knowles NR (2003) Wound-induced superoxide production and PAL activity decline with potato tuber age and wound healing ability. Physiol Plant 117:108–117
Kumar GNM, Iyer S, Knowles NR (2007) Extraction of RNA from fresh, frozen, and lyophilized tuber and root tissues. J. Agric Food Chem 55:1674–1678
Kumar GNM, Knowles NR (2014) Wound response is activated in tubers infected with zebra chip. In: Proceedings of the 97th annual meeting of the Potato Association of America. Am J Pot Res 91:51–52
Kumar GNM, Knowles LO, Knowles NR (2015) Zebra chip infection enhances tuber respiration and oxidative stress. In: Proceedings of the 99th annual meeting of the Potato Association of America. Am J Pot Res (in press)
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nat 227:680–685
Laxalt AM, Munnik T (2002) Phospholipid signalling in plant defense. Curr Opinion Plant Biol 5:332–338
Lisón P, Rodrigo I, Conejero V (2006) A novel function for the cathepsin D inhibitor in tomato. Plant Physiol 142:1329–1339
Liu YW, Han CH, Lee MH, Hsu FL, Hou WC (2003) Patatin, the tuber storage protein of potato (Solanum tuberosum L.), exhibits antioxidant activity in vitro. J Agric Food Chem 16:4389–4393
Mareš M, Meloun B, Pavlík M, Kostka V, Baudyš M (1989) Primary structure of cathepsin D inhibitor from potatoes and its structure relationship to soybean trypsin inhibitor family. FEBS Lett 251:94–98
Michaud D, Faye L, Yelle S (1993) Electrophoretic analysis of plant cystein and serine proteinases using gelatin-containing polyacrylamide gels and class-specific proteinase inhibitors. Electrophoresis 14:94–98
Michaud D, Nguyen-Quoc B, Bernier-Vadnais N, Faye L, Yelle S (2006) Cysteine proteinase forms in sprouting potato tuber. Physiol Plant 90:497–503
Munnik T, Irvine RF, Musgrav A (1998) Phospholipid signaling in plants. Biochim Biophys Acta 1389:222–272
Munyaneza JE, Crosslin JM, Upton JE (2007a) Association of Bactericera cockerelli (Homoptera: Psyllidae) with “zebra-chip,” a new potato disease in southwestern United States and Mexico. J Econ Entomol 100(3):656–663
Munyaneza JE, Goolsby JA, Crosslin JM, Upton JE (2007b) Further evidence that zebra-chip potato disease in the lower Rio Grande Valley of Texas is associated with Bactericera cockerelli. Subtropical Plant Sci 59:30–37
Navarre DA, Shakya R, Holden J, Crosslin JM (2009) LC-MS analysis of phenolic compounds in tubers showing zebra chip symptoms. Am J Pot Res 86:88–95
Nissen MS, Kumar GNM, Youn B, Knowles DB, Lam KS, Ballinger WJ, Knowles NR, Kang C (2009) Characterization of potato multicystatin and its structural comparison with other cystatins. Plant Cell 21:861–875
Ohara-Takada A, Matsuura-Endo C, Chuda Y, Ono H, Yada H, Yoshida M, Kodayashi A, Tsuda S, Takigawa S, Noda T, Yamauchi H, Mori M (2005) Change in content of sugars and free amino acids in potato tubers under short-term storage at low temperature and the effect on acrylamide level after frying. Biosci Biotech Biochem 69:1232–1238
Orr G, Strickland J, Walsh T (1994) Inhibition of Diabrotica larval growth by a multicystatin from potato tubers. J Insect Physiol 40:893–900
Park Y, Choi BH, Kwak SJ, Kang CW, Lim HT, Cheong HS, Hahm KS (2005) Kunitz-type serine protease inhibitor from potato (Solanum tuberosum L. cv. Jopung). J Agric Food Chem 53:6491–6496
Peňa-Cortéz H, Fisahn J, Willmitzer L (1995) Signals involved in wound-induced proteinase inhibitor II gene expression in tomato and potato plants. Proc Natl Acad Sci 92:4106–4113
Pouvreau L, Gruppen H, Piersma SR, Van der Broek LAM, van Koningsveld GA, Voragen AGJ (2001) Relative abundance and inhibitory distribution of protease inhibitors in potato juice from cv. Elkana. J Agric Food Chem 49:2864–2874
Pouvreau L, Gruppen H, van Koningsveld GA, van den Broek LAM, Voragen AGJ (2003) The most abundant protease inhibitor in potato tuber (Cv. Elkana) is a serine-protease inhibitor from the kunitz family. J Agric Food Chem 51:5001–5005
Prat S, Frommer WB, Höfgen R, Keil M, Kossmann J, Köster-Töpfer M, Liu X-J, Müller B, Peña-Cortés H, Rocha-Sosa M, Sánchez-Serrano JJ, Sonnewald U, Willmitzer L (1990) Gene expression during tuber development in potato plants. FEBS Lett 268:334–338
Racusen D (1984) Lipid acyl hydrolase of patatin. Can J Bot 62:1640–1644
Racusen D, Foote M (1980) A major soluble glycoprotein of potato tubers. J Food Biochem 4:43–52
Rashed A, Wallis CM, Paetzold L, Workneh F, Rush CM (2013) Zebra-chip disease and potato biochemistry: tuber physiological changes in response to ‘Candidatus Liberibacter solanacearum’ infection over time. Phytopath 103:419–426
Ravindran A, Levy J, Pierson E, Gross DC (2011) Development of primers for improved PCR detection of the potato zebra-chip pathogen, ‘Candidatus Liberibacter solanacearum’. Plant Dis 95:1542–1546
Revina TA, Kladnitskaya GV, Gerasimova NG, Gvozdeva EL, Valueva TA (2010) Protein trypsin inhibitor from potato tubers. Biochem (Moscow) 75:36–40
Rodis P, Hoff JE (1984) Naturally occurring protein crystals in the Potato. Inhibitor of papain, chymopapain, and ficin. Plant Physiol 74: 907–911
Rondon S, Schreiber A, Jensen A, Hamm P, Munyaneza JE, Nolte, P, Olsen N, Wenninger E, Henne D, Wohleb C, Waters T (2012) Potato psillid vector of zebra chip disease in the Pacific Northwest: biology, ecology, and management. Oregon State University Extension Bulletin 633. http://ir.library.oregonstate.edu/xmlui/bitstream/handle/1957/30058/pnw633.pdf
Rosahl S, Schell J, Willmitzer L (1987) Expression of a tuber specific storage protein in transgenic tobacco plants; demonstration of an esterase activity. EMBO J 6:1155–1159
Ryan CA (1990) Protease inhibitors in plants. Genes for improving defenses against insects and pathogens. Ann Rev Phytopath 28:425–449
Schaller A (2004) A cut above the rest: the regulatory function of plant proteases: review. Planta 220:183–197
Schlüter U, Benchabane M, Munger A, Kiggundu A, Vorster J, Goulet MC, Cloutier MC, Michaud D (2010) Recombinant protease inhibitors for herbivore pest control: a multitrophic perspective. J Expt Bot 61:4169–4183
Senda K, Yoshioka H, Doke N, Kawakita KA (1996) Cytosolic phospholipase A2 from potato tissues appears to be patatin. Plant Cell Physiol 37:347–353
Simões I, Faro C (2004) Structure and function of plant aspartic proteinases. Eur J Biochem 271:2067–2075
Simões I, Faro R, Bur D, Faro C (2007) Characterization of recombinant CDR1, an Arabidopsis aspartic proteinase involved in disease resistance. J Biol Chem 282:31358–31365
van der Hoorn RAL (2008) Plant proteases: from phenotypes to molecular mechanisms. Annu Rev Plant Biol 59:191–223
van der Hoorn RAL, Jones JD (2004) The plant proteolytic machinery and its role in defense. Curr Opin Plant Biol 7:400–407
van der Hoorn RAL, Leeuwenburgh MA, Bogyo M, Joosten MHA, Peck SC (2004) Activity profiling of papain-like cysteine proteases in plants. Plant Physiol 135:1170–1178
Vierstra RD (1996) Proteolysis in plants: mechanisms and functions. Plant Mol Biol 32:275–302
Wallis CM, Chen J, Civerolo EL (2012) Zebra-chip-diseased potato tubers are characterized by increased levels of host phenolics, amino acids, and defense-related proteins. Physiol Mol Plant Path 78:66–72
Wallis CM, Rashed A, Wallingford AK, Paetzold L, Workneh F, Rush CM (2014) Similarities and differences in physiological responses to ‘Candidatus Liberibacter solanacearum’ infection among different potato cultivars. Phytopathology 104:126–133
Wallis CM, Rashed A, Chen J, Paetzold L, Workneh F, Rush CM (2015) Effects of potato-psyllid-vectored ‘Candidatus Liberibacter solanacearum’ infection on potato leaf and stem physiology. Phytopathology 105:189–198
Walsh TA, Twitchell WP (1991) Two Kunitz-type proteinase inhibitors from potato tubers. Plant Physiol 97:15–18
Weeda SM, Kumar GNM, Knowles NR (2009) Developmentally linked changes in proteases and protease inhibitors suggest a role for potato multicystatin in regulating protein content of potato tubers. Planta 230:73–84
Weeda SM, Kumar GNM, Knowles NR (2011) Protein mobilization from potato tubers during long-term storage and daughter tuber formation. Intl J Plant Sci 172:459–470
Xia Y, Suzuki H, Borevitz J, Blount J, Guo Z, Patel K, Dixon RA, Lamb C (2004) An extracellular aspartic protease functions in Arabidopsis disease resistance signaling. EMBO J 23(4):980–988
Acknowledgments
We thank Dr. Joseph E. Munyaneza (USDA-ARS, Wapato, WA) for providing tuber samples. Financial support was provided by the USDA-ARS, Washington State Potato Commission, and Washington State University Agricultural Research Center to N.R. Knowles.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kumar, G.N.M., Knowles, L.O. & Knowles, N.R. Zebra chip disease decreases tuber (Solanum tuberosum L.) protein content by attenuating protease inhibitor levels and increasing protease activities. Planta 242, 1153–1166 (2015). https://doi.org/10.1007/s00425-015-2346-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2346-9