Abstract
Main conclusion
Differential palisade and spongy parenchyma structural changes in oilseed rape leaf were demonstrated. These dismantling processes were linked to early senescence events and associated to remobilization processes.
Abstract
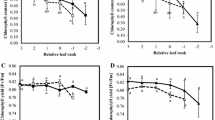
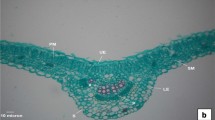
During leaf senescence, an ordered cell dismantling process allows efficient nutrient remobilization. However, in Brassica napus plants, an important amount of nitrogen (N) in fallen leaves is associated with low N remobilization efficiency (NRE). The leaf is a complex organ mainly constituted of palisade and spongy parenchyma characterized by different structures and functions concerning water relations and carbon fixation. The aim of the present study was to demonstrate a specific structural evolution of these parenchyma throughout natural senescence in B. napus, probably linked to differential nutrient remobilization processes. The study was performed on 340 leaves from 32 plants during an 8-week development period under controlled growing conditions. Water distribution and status at the cellular level were investigated by low-field proton nuclear magnetic resonance (NMR), while light and electron microscopy were used to observe cell and plast structure. Physiological parameters were determined on all leaves studied and used as indicators of leaf development and remobilization progress. The results revealed a process of hydration and cell enlargement of leaf tissues associated with senescence. Wide variations were observed in the palisade parenchyma while spongy cells changed only very slightly. The major new functional information revealed was the link between the early senescence events and specific tissue dismantling processes.









Similar content being viewed by others
References
Albert B, Le Caherec F, Niogret MF, Faes P, Avice JC, Leport L, Bouchereau A (2012) Nitrogen availability impacts oilseed rape (Brassica napus L.) plant water status and proline production efficiency under water-limited conditions. Planta 236:659–676
Avice J-C, Etienne P (2014) Leaf senescence and nitrogen remobilization efficiency in oilseed rape (Brassica napus L.). J Exp Bot 65(14):3813–3824
Bacelar EA, Santos DL, Moutinho-Pereira JM, Goncalves BC, Ferreira HF, Correia CM (2006) Immediate responses and adaptative strategies of three olive cultivars under contrasting water availability regimes: changes on structure and chemical composition of foliage and oxidative damage. Plant Sci 170:596–605
Bohler S, Sergeant K, Jolivet Y, Hoffmann L, Hausman J-F, Dizengremel P, Renaut J (2013) A physiological and proteomic study of poplar leaves during ozone exposure combined with mild drought. Proteomics 13:1737–1754
Brehelin C, Kessler F, van Wijk KJ (2007) Plastoglobules: versatile lipoprotein particles in plastids. Trends Plant Sci 12:260–266
BuchananWollaston V (1997) The molecular biology of leaf senescence. J Exp Bot 48:181–199
Capitani D, Brilli F, Mannina L, Proietti N, Loreto F (2009) In situ investigation of leaf water status by portable unilateral nuclear magnetic resonance. Plant Physiol 149:1638–1647
Castro-Diez P, Puyravaud JP, Cornelissen JHC (2000) Leaf structure and anatomy as related to leaf mass per area variation in seedlings of a wide range of woody plant species and types. Oecologia 124:476–486
Colire C, Lerumeur E, Gallier J, Decertaines J, Larher F (1988) An assessment of proton nuclear magnetic-resonance as an alternative method to describe water status of leaf tissues in wilted plants. Plant Physiol Biochem 26:767–776
Diaz C, Lemaitre T, Christ A, Azzopardi M, Kato Y, Sato F, Morot-Gaudry J-F, Le Dily F, Masclaux-Daubresse C (2008) Nitrogen recycling and remobilization are differentially controlled by leaf senescence and development stage in Arabidopsis under low nitrogen nutrition. Plant Physiol 147:1437–1449
Ghosh S, Mahoney SR, Penterman JN, Peirson D, Dumbroff EB (2001) Ultrastructural and biochemical changes in chloroplasts during Brassica napus senescence. Plant Physiol Biochem 39:777–784
Gombert J, Etienne P, Ourry A, Le Dily F (2006) The expression patterns of SAG12/Cab genes reveal the spatial and temporal progression of leaf senescence in Brassica napus L. with sensitivity to the environment. J Exp Bot 57:1949–1956
Gotow K, Taylor S, Zeiger E (1988) Photosynthetic carbon fixation in guard-cell protoplasts of vicia-fabia L—evidence from radiolabel experiments. Plant Physiol 86:700–705
Guiboileau A, Yoshimoto K, Soulay F, Bataille M-P, Avice J-C, Masclaux-Daubresse C (2012) Autophagy machinery controls nitrogen remobilization at the whole-plant level under both limiting and ample nitrate conditions in Arabidopsis. New Phytol 194:732–740
Günthardt-Goerg M, McQuattie C, Maurer S, Frey B (2000) Visible and microscopic injury in leaves of five deciduous tree species related to current critical ozone levels. Environ Pollut 109:489–500
Hills BP, Remigereau B (1997) NMR studies of changes in subcellular water compartmentation in parenchyma apple tissue during drying and freezing. Int J Food Sci Technol 32:51–61
Hoertensteiner S (2006) Chlorophyll degradation during senescence. Annu Rev Plant Biol 57:55–77
Inada N, Sakai A, Kuroiwa H, Kuroiwa T (1998) Three-dimensional analysis of the senescence program in rice (Oryza sativa L.) coleoptiles—Investigations by fluorescence microscopy and electron microscopy. Planta 206:585–597
Keech O, Pesquet E, Ahad A, Askne A, Nordvall D, Vodnala SM, Tuominen H, Hurry V, Dizengremel P, Gardestroem P (2007) The different fates of mitochondria and chloroplasts during dark-induced senescence in Arabidopsis leaves. Plant Cell Environ 30:1523–1534
Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58:115–136
Mae T, Ohira K (1981) The remobilization of nitrogen related to leaf growth and senescence in rice Oryza sativa (L). Plant Cell Physiol 22:1067–1074
Makino A, Osmond B (1991) Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiol 96:355–362
Martinez JP, Silva H, Ledent JF, Pinto M (2007) Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur J Agron 26:30–38
Masclaux C, Valadier M-H, Brugière N, Morot-Gaudry J-F, Hirel B (2000) Characterization of the sink/source transition in tobacco Nicotiana tabacum (L.) shoots in relation to nitrogen management and leaf senescence. Planta 211:510–518
Masclaux-Daubresse C, Reisdorf-Cren M, Orsel M (2008) Leaf nitrogen remobilisation for plant development and grain filling. Plant Biology 10:23–36
McCain DC (1995) Nuclear magnetic resonance study of spin relaxation and magnetic field gradients in maple leaves. Biophys J 69:1111–1116
McIntyre GI (1987) The role of water in the regulation of plant development. Canadian J Botany-Revue Canadienne De Botanique 65:1287–1298
Mohapatra PK, Patro L, Raval MK, Ramaswamy NK, Biswal UC, Biswal B (2010) Senescence-induced loss in photosynthesis enhances cell wall beta-glucosidase activity. Physiol Plant 138:346–355
Musse M, De Franceschi L, Cambert M, Sorin C, Le Caherec F, Burel A, Bouchereau A, Mariette F, Leport L (2013) Structural changes in Senescing oilseed rape leaves at tissue and subcellular levels monitored by nuclear magnetic resonance relaxometry through water status. Plant Physiol 163:392–406
Nardini A, Raimondo F, Lo Gullo MA, Salleo S (2010) Leafminers help us understand leaf hydraulic design. Plant Cell Environ 33:1091–1100
Nooden LD, Guiamet JJ, John I (1997) Senescence mechanisms. Physiol Plant 101:746–753
Otegui MS, Noh YS, Martinez DE, Vila Petroff MG, Andrew Staehelin L, Amasino RM, Guiamet JJ (2005) Senescence-associated vacuoles with intense proteolytic activity develop in leaves of Arabidopsis and soybean. Plant J 41:831–844
Parthier B (1988) Gerontoplasts—the yellow end in the ontogenesis of chloroplasts. Int J Endocytobiosis Cell Res 5:163–190
Sakamoto W (2006) Protein degradation machineries in plastids. Annu Rev Plant Biol 57:599–621
Snaar JEM, Van As H (1992a) A method for the simultaneous measurement of NMR spin-lattice and spin-spin relaxation times in compartmentalized systems. J Magn Reson 99:139–148
Snaar JEM, Van As H (1992b) Probing water compartments and membrane permeability in plant cells by 1H NMR relaxation measurements. Biophys J 63:1654–1658
Thompson JE, Froese CD, Madey E, Smith MD, Hong YW (1998) Lipid metabolism during plant senescence. Prog Lipid Res 37:119–141
Van As H (2007) Intact plant MRI for the study of cell water relations, membrane permeability, cell-to-cell and long distance water transport. J Exp Bot 58:743–756
van der Weerd L, Claessens M, Ruttink T, Vergeldt FJ, Schaafsma TJ, Van As H (2001) Quantitative NMR microscopy of osmotic stress responses in maize and pearl millet. J Exp Bot 52:2333–2343
Wada S, Ishida H (2009) Chloroplasts autophagy during senescence of individually darkened leaves. Plant Signal Behav 4:565–567
Wuyts N, Massonnet C, Dauzat M, Granier C (2012) Structural assessment of the impact of environmental constraints on Arabidopsis thaliana leaf growth: a 3D approach. Plant Cell Environ 35:1631–1646
Zhang K, Xia X, Zhang Y, Gan S-S (2012) An ABA-regulated and Golgi-localized protein phosphatase controls water loss during leaf senescence in Arabidopsis. Plant J 69:667–678
Zwieniecki MA, Brodribb TJ, Holbrook NM (2007) Hydraulic design of leaves: insights from rehydration kinetics. Plant Cell Environ 30:910–921
Acknowledgments
We thank the Regional Council of Bretagne for financial support. We also thank the Genetic Resources Center (BrACySol, BRC, UMR IGEPP, INRA Ploudaniel, France) for providing the seeds of the Tenor variety. We thank our colleagues of the Biopolymers, Structural Biology platform, INRA Nantes, France for their help and support with TEM studies. We thank Mireille CAMBERT (IRSTEA) for her assistance with NMR measurements, Françoise LEPRINCE for starch analysis, and Patrick LECONTE and the greenhouse team (IGEPP) for technical support with plant management.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sorin, C., Musse, M., Mariette, F. et al. Assessment of nutrient remobilization through structural changes of palisade and spongy parenchyma in oilseed rape leaves during senescence. Planta 241, 333–346 (2015). https://doi.org/10.1007/s00425-014-2182-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-014-2182-3