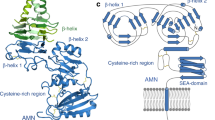
Abstract
Megalin (or LRP2) is an endocytic receptor that plays a central role in embryonic development and adult tissue homeostasis. Loss of this receptor in congenital or acquired diseases results in multiple organ dysfunctions, including forebrain malformation (holoprosencephaly) and renal reabsorption defects (renal Fanconi syndrome). Here, we describe current concepts of the mode of receptor action that include co-receptors and a repertoire of different ligands, and we discuss how these interactions govern functional integrity of the kidney and the brain, and cause disease when defective.




Similar content being viewed by others
References
Aminoff M, Carter JE, Chadwick RB, Johnson C, Grasbeck R, Abdelaal MA, Broch H, Jenner LB, Verroust PJ, Moestrup SK, de la Chapelle A, Krahe R (1999) Mutations in CUBN, encoding the intrinsic factor-vitamin B12 receptor, cubilin, cause hereditary megaloblastic anaemia 1. Nat Genet 21:309–313
Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, Nexo E, Verroust PJ, Christensen EI, Kozyraki R (2010) Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol 21:1859–1867. doi:10.1681/ASN.2010050492
Anzenberger U, Bit-Avragim N, Rohr S, Rudolph F, Dehmel B, Willnow TE, Abdelilah-Seyfried S (2006) Elucidation of megalin/LRP2-dependent endocytic transport processes in the larval zebrafish pronephros. J Cell Sci 119:2127–2137. doi:10.1242/jcs.02954
Argraves WS, Morales CR (2004) Immunolocalization of cubilin, megalin, apolipoprotein J, and apolipoprotein A-I in the uterus and oviduct. Mol Reprod Dev 69:419–427. doi:10.1002/mrd.20174
Attree O, Olivos IM, Okabe I, Bailey LC, Nelson DL, Lewis RA, McInnes RR, Nussbaum RL (1992) The Lowe’s oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 358:239–242. doi:10.1038/358239a0
Bachmann S, Schlichting U, Geist B, Mutig K, Petsch T, Bacic D, Wagner CA, Kaissling B, Biber J, Murer H, Willnow TE (2004) Kidney-specific inactivation of the megalin gene impairs trafficking of renal inorganic sodium phosphate cotransporter (NaPi-IIa). J Am Soc Nephrol 15:892–900
Biemesderfer D, Nagy T, DeGray B, Aronson PS (1999) Specific association of megalin and the Na+/H+ exchanger isoform NHE3 in the proximal tubule. J Biol Chem 274:17518–17524
Biemesderfer D, DeGray B, Aronson PS (2001) Active (9.6 s) and inactive (21 s) oligomers of NHE3 in microdomains of the renal brush border. J Biol Chem 276:10161–10167
Birn H, Willnow TE, Nielsen R, Norden AG, Bonsch C, Moestrup SK, Nexo E, Christensen EI (2002) Megalin is essential for renal proximal tubule reabsorption and accumulation of transcobalamin-B(12). Am J Physiol Renal Physiol 282:F408–F416. doi:10.1152/ajprenal.00206.2000
Briscoe J, Therond PP (2013) The mechanisms of hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol 14:416–429. doi:10.1038/nrm3598
Burmeister R, Boe IM, Nykjaer A, Jacobsen C, Moestrup SK, Verroust P, Christensen EI, Lund J, Willnow TE (2001) A two-receptor pathway for catabolism of Clara cell secretory protein in the kidney. J Biol Chem 276:13295–13301. doi:10.1074/jbc.M010679200
Cases O, Joseph A, Obry A, Santin MD, Ben-Yacoub S, Paques M, Amsellem-Levera S, Bribian A, Simonutti M, Augustin S, Debeir T, Sahel JA, Christ A, de Castro F, Lehericy S, Cosette P, Kozyraki R (2015) Foxg1-Cre mediated Lrp2 inactivation in the developing mouse neural retina, ciliary and retinal pigment epithelia models congenital high myopia. PLoS One 10:e0129518. doi:10.1371/journal.pone.0129518
Chasman DI, Fuchsberger C, Pattaro C et al (2012) Integration of genome-wide association studies with biological knowledge identifies six novel genes related to kidney function. Hum Mol Genet 21:5329–5343. doi:10.1093/hmg/dds369
Chatelet F, Brianti E, Ronco P, Roland J, Verroust P (1986) Ultrastructural localization by monoclonal antibodies of brush border antigens expressed by glomeruli. II. Extrarenal distribution. Am J Pathol 122:512–519
Chen J, Chen JK, Conway EM, Harris RC (2013) Survivin mediates renal proximal tubule recovery from AKI. J Am Soc Nephrol 24:2023–2033. doi:10.1681/ASN.2013010076
Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA (1996) Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 383:407–413. doi:10.1038/383407a0
Cho SH, Cepko CL (2006) Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development 133:3167–3177. doi:10.1242/dev.02474
Christ A, Christa A, Kur E, Lioubinski O, Bachmann S, Willnow TE, Hammes A (2012) LRP2 is an auxiliary SHH receptor required to condition the forebrain ventral midline for inductive signals. Dev Cell 22:268–278. doi:10.1016/j.devcel.2011.11.023
Christ A, Christa A, Klippert J, Eule JC, Bachmann S, Wallace VA, Hammes A, Willnow TE (2015) LRP2 acts as SHH clearance receptor to protect the retinal margin from mitogenic stimuli. Dev Cell 35:36–48. doi:10.1016/j.devcel.2015.09.001
Christensen EI, Nielsen S, Moestrup SK, Borre C, Maunsbach AB, de Heer E, Ronco P, Hammond TG, Verroust P (1995) Segmental distribution of the endocytosis receptor gp330 in renal proximal tubules. Eur J Cell Biol 66:349–364
Christensen EI, Moskaug JO, Vorum H, Jacobsen C, Gundersen TE, Nykjaer A, Blomhoff R, Willnow TE, Moestrup SK (1999) Evidence for an essential role of megalin in transepithelial transport of retinol. J Am Soc Nephrol 10:685–695
Christensen EI, Verroust PJ, Nielsen R (2009) Receptor-mediated endocytosis in renal proximal tubule. Pflugers Arch 458:1039–1048. doi:10.1007/s00424-009-0685-8
Dachy A, Paquot F, Debray G, Bovy C, Christensen EI, Collard L, Jouret F (2015) In-depth phenotyping of a Donnai-Barrow patient helps clarify proximal tubule dysfunction. Pediatr Nephrol 30:1027–1031. doi:10.1007/s00467-014-3037-7
Dagil R, O’Shea C, Nykjaer A, Bonvin AM, Kragelund BB (2013) Gentamicin binds to the megalin receptor as a competitive inhibitor using the common ligand binding motif of complement type repeats: insight from the nmr structure of the 10th complement type repeat domain alone and in complex with gentamicin. J Biol Chem 288:4424–4435. doi:10.1074/jbc.M112.434159
de Jong M, Barone R, Krenning E, Bernard B, Melis M, Visser T, Gekle M, Willnow TE, Walrand S, Jamar F, Pauwels S (2005) Megalin is essential for renal proximal tubule reabsorption of (111)In-DTPA-octreotide. J Nucl Med 46:1696–1700
Eshbach ML, Weisz OA (2017) Receptor-mediated endocytosis in the proximal tubule. Annu Rev Physiol 79:425–448. doi:10.1146/annurev-physiol-022516-034234
Fass D, Blacklow S, Kim PS, Berger JM (1997) Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature 388:691–693. doi:10.1038/41798
Gajera CR, Emich H, Lioubinski O, Christ A, Beckervordersandforth-Bonk R, Yoshikawa K, Bachmann S, Christensen EI, Gotz M, Kempermann G, Peterson AS, Willnow TE, Hammes A (2010) LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci 123:1922–1930. doi:10.1242/jcs.065912
Gallagher H, Oleinikov AV, Fenske C, Newman DJ (2004) The adaptor disabled-2 binds to the third PsixNPxY sequence on the cytoplasmic tail of megalin. Biochimie 86:179–182
Gburek J, Verroust PJ, Willnow TE, Fyfe JC, Nowacki W, Jacobsen C, Moestrup SK, Christensen EI (2002) Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J Am Soc Nephrol 13:423–430
Gburek J, Birn H, Verroust PJ, Goj B, Jacobsen C, Moestrup SK, Willnow TE, Christensen EI (2003) Renal uptake of myoglobin is mediated by the endocytic receptors megalin and cubilin. Am J Physiol Ren Physiol 285:F451–F458
Goetz SC, Anderson KV (2010) The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11:331–344. doi:10.1038/nrg2774
Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J (2000) Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem 275:25616–25624. doi:10.1074/jbc.M000955200
Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert FJ, Luppa PB, Nykjaer A, Willnow TE (2005) Role of endocytosis in cellular uptake of sex steroids. Cell 122:751–762. doi:10.1016/j.cell.2005.06.032
Hilpert J, Wogensen L, Thykjaer T, Wellner M, Schlichting U, Orntoft TF, Bachmann S, Nykjaer A, Willnow TE (2002) Expression profiling confirms the role of endocytic receptor megalin in renal vitamin D3 metabolism. Kidney Int 62:1672–1681. doi:10.1046/j.1523-1755.2002.00634.x
Hoch RV, Rubenstein JL, Pleasure S (2009) Genes and signaling events that establish regional patterning of the mammalian forebrain. Semin Cell Dev Biol 20:378–386. doi:10.1016/j.semcdb.2009.02.005
Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N (2005) The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett 579:773–777. doi:10.1016/j.febslet.2004.12.031
Jobst-Schwan T, Knaup KX, Nielsen R, Hackenbeck T, Buettner-Herold M, Lechler P, Kroening S, Goppelt-Struebe M, Schloetzer-Schrehardt U, Furnrohr BG, Voll RE, Amann K, Eckardt KU, Christensen EI, Wiesener MS (2013) Renal uptake of the antiapoptotic protein survivin is mediated by megalin at the apical membrane of the proximal tubule. Am J Physiol Ren Physiol 305:F734–F744. doi:10.1152/ajprenal.00546.2012
Kantarci S, Al-Gazali L, Hill RS, Donnai D, Black GC, Bieth E, Chassaing N, Lacombe D, Devriendt K, Teebi A, Loscertales M, Robson C, Liu T, MacLaughlin DT, Noonan KM, Russell MK, Walsh CA, Donahoe PK, Pober BR (2007) Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat Genet 39:957–959
Kerjaschki D, Farquhar MG (1982) The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A 79:5557–5561
Keyel PA, Thieman JR, Roth R, Erkan E, Everett ET, Watkins SC, Heuser JE, Traub LM (2008) The AP-2 adaptor beta2 appendage scaffolds alternate cargo endocytosis. Mol Biol Cell 19:5309–5326. doi:10.1091/mbc.E08-07-0712
Klahr S (2003) The bone morphogenetic proteins (BMPs). Their role in renal fibrosis and renal function. J Nephrol 16:179–185
Klassen RB, Allen PL, Batuman V, Crenshaw K, Hammond TG (1985) Light chains are a ligand for megalin. J Appl Physiol 98:257–263. doi:10.1152/japplphysiol.01090.2003
Kozyraki R, Fyfe J, Verroust PJ, Jacobsen C, Dautry-Varsat A, Gburek J, Willnow TE, Christensen EI, Moestrup SK (2001) Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc Natl Acad Sci U S A 98:12491–12496. doi:10.1073/pnas.211291398
Kur E, Christa A, Veth KN, Gajera CR, Andrade-Navarro MA, Zhang J, Willer JR, Gregg RG, Abdelilah-Seyfried S, Bachmann S, Link BA, Hammes A, Willnow TE (2011) Loss of Lrp2 in zebrafish disrupts pronephric tubular clearance but not forebrain development. Dev Dyn 240:1567–1577. doi:10.1002/dvdy.22624
Kur E, Mecklenburg N, Cabrera RM, Willnow TE, Hammes A (2014) LRP2 mediates folate uptake in the developing neural tube. J Cell Sci 127:2261–2268. doi:10.1242/jcs.140145
Lamba D, Karl M, Reh T (2008) Neural regeneration and cell replacement: a view from the eye. Cell Stem Cell 2:538–549. doi:10.1016/j.stem.2008.05.002
Larsson M, Hjalm G, Sakwe AM, Engstrom A, Hoglund AS, Larsson E, Robinson RC, Sundberg C, Rask L (2003) Selective interaction of megalin with postsynaptic density-95 (PSD-95)-like membrane-associated guanylate kinase (MAGUK) proteins. Biochem J 373:381–391
Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE (1999) Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol 155:1361–1370. doi:10.1016/S0002-9440(10)65238-8
Li Y, van Kerkhof P, Marzolo MP, Strous GJ, Bu G (2001) Identification of a major cyclic AMP-dependent protein kinase A phosphorylation site within the cytoplasmic tail of the low-density lipoprotein receptor-related protein: implication for receptor-mediated endocytosis. Mol Cell Biol 21:1185–1195. doi:10.1128/MCB.21.4.1185-1195.2001
Li Y, Cong R, Biemesderfer D (2008) The COOH terminus of megalin regulates gene expression in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol 295:C529–C537. doi:10.1152/ajpcell.00037.2008
Lloyd SE, Pearce SH, Fisher SE, Steinmeyer K, Schwappach B, Scheinman SJ, Harding B, Bolino A, Devoto M, Goodyer P, Rigden SP, Wrong O, Jentsch TJ, Craig IW, Thakker RV (1996) A common molecular basis for three inherited kidney stone diseases. Nature 379:445–449. doi:10.1038/379445a0
Lou X, McQuistan T, Orlando RA, Farquhar MG (2002) GAIP, GIPC and Galphai3 are concentrated in endocytic compartments of proximal tubule cells: putative role in regulating megalin’s function. J Am Soc Nephrol 13:918–927
Lundgren S, Carling T, Hjalm G, Juhlin C, Rastad J, Pihlgren U, Rask L, Akerstrom G, Hellman P (1997) Tissue distribution of human gp330/megalin, a putative Ca(2+)-sensing protein. J Histochem Cytochem 45:383–392
Mahadevappa R, Nielsen R, Christensen EI, Birn H (2014) Megalin in acute kidney injury: foe and friend. Am J Physiol Ren Physiol 306:F147–F154. doi:10.1152/ajprenal.00378.2013
Marino M, Lisi S, Pinchera A, Chiovato L, McCluskey RT (2003) Targeting of thyroglobulin to transcytosis following megalin-mediated endocytosis: evidence for a preferential pH-independent pathway. J Endocrinol Investig 26:222–229
Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P (2004) Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 15:3073–3082. doi:10.1097/01.ASN.0000145013.44578.45
Moestrup SK, Cui S, Vorum H, Bregengard C, Bjorn SE, Norris K, Gliemann J, Christensen EI (1995) Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J Clin Invest 96:1404–1413
Moestrup SK, Kozyraki R, Kristiansen M, Kaysen JH, Rasmussen HH, Brault D, Pontillon F, Goda FO, Christensen EI, Hammond TG, Verroust PJ (1998) The intrinsic factor-vitamin B12 receptor and target of teratogenic antibodies is a megalin-binding peripheral membrane protein with homology to developmental proteins. J Biol Chem 273:5235–5242
Morales CR, Zeng J, El Alfy M, Barth JL, Chintalapudi MR, McCarthy RA, Incardona JP, Argraves WS (2006) Epithelial trafficking of Sonic hedgehog by megalin. J Histochem Cytochem 54:1115–1127. doi:10.1369/jhc.5A6899.2006
Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D’Agati V, Devarajan P, Barasch J (2005) Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 115:610–621. doi:10.1172/JCI23056
Morris SM, Tallquist MD, Rock CO, Cooper JA (2002) Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J 21:1555–1564
Muenke M, Beachy PA (2000) Genetics of ventral forebrain development and holoprosencephaly. Curr Opin Genet Dev 10:262–269
Naccache SN, Hasson T, Horowitz A (2006) Binding of internalized receptors to the PDZ domain of GIPC/synectin recruits myosin VI to endocytic vesicles. Proc Natl Acad Sci U S A 103:12735–12740. doi:10.1073/pnas.0605317103
Nagai M, Meerloo T, Takeda T, Farquhar MG (2003) The adaptor protein ARH escorts megalin to and through endosomes. Mol Biol Cell 14:4984–4996
Nagai J, Christensen EI, Morris SM, Willnow TE, Cooper JA, Nielsen R (2005) Mutually dependent localization of megalin and Dab2 in the renal proximal tubule. Am J Physiol Ren Physiol 289:F569–F576. doi:10.1152/ajprenal.00292.2004
Nielsen R, Courtoy PJ, Jacobsen C, Dom G, Lima WR, Jadot M, Willnow TE, Devuyst O, Christensen EI (2007) Endocytosis provides a major alternative pathway for lysosomal biogenesis in kidney proximal tubular cells. Proc Natl Acad Sci U S A 104:5407–5412. doi:10.1073/pnas.0700330104
Nielsen R, Christensen EI, Birn H (2016) Megalin and cubilin in proximal tubule protein reabsorption: from experimental models to human disease. Kidney Int 89:58–67. doi:10.1016/j.kint.2015.11.007
Nykjaer A, Willnow TE (2002) The low-density lipoprotein receptor gene family: a cellular Swiss army knife? Trends Cell Biol 12:273–280
Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE (1999) An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96:507–515
Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI (2001) Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc Natl Acad Sci U S A 98:13895–13900
Oleinikov AV, Zhao J, Makker SP (2000) Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochem J 347(Pt 3):613–621
Orlando RA, Rader K, Authier F, Yamazaki H, Posner BI, Bergeron JJ, Farquhar MG (1998) Megalin is an endocytic receptor for insulin. J Am Soc Nephrol 9:1759–1766
Ortega MC, Cases O, Merchan P, Kozyraki R, Clemente D, de Castro F (2012) Megalin mediates the influence of sonic hedgehog on oligodendrocyte precursor cell migration and proliferation during development. Glia 60:851–866. doi:10.1002/glia.22316
Petersen HH, Hilpert J, Militz D, Zandler V, Jacobsen C, Roebroek AJ, Willnow TE (2003) Functional interaction of megalin with the megalinbinding protein (MegBP), a novel tetratrico peptide repeat-containing adaptor molecule. J Cell Sci 116:453–461
Piwon N, Gunther W, Schwake M, Bosl MR, Jentsch TJ (2000) ClC-5 Cl--channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature 408:369–373
Raila J, Willnow TE, Schweigert FJ (2005) Megalin-mediated reuptake of retinol in the kidneys of mice is essential for vitamin A homeostasis. J Nutr 135:2512–2516
Rudenko G, Henry L, Henderson K, Ichtchenko K, Brown MS, Goldstein JL, Deisenhofer J (2002) Structure of the LDL receptor extracellular domain at endosomal pH. Science 298:2353–2358
Saito A, Pietromonaco S, Loo AK, Farquhar MG (1994) Complete cloning and sequencing of rat gp330/“megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci U S A 91:9725–9729
Schmitz C, Hilpert J, Jacobsen C, Boensch C, Christensen EI, Luft FC, Willnow TE (2002) Megalin deficiency offers protection from renal aminoglycoside accumulation. J Biol Chem 277:618–622
Seetharam B, Christensen EI, Moestrup SK, Hammond TG, Verroust PJ (1997) Identification of rat yolk sac target protein of teratogenic antibodies, gp280, as intrinsic factor-cobalamin receptor. J Clin Invest 99:2317–2322
Shah M, Baterina OY Jr, Taupin V, Farquhar MG (2013) ARH directs megalin to the endocytic recycling compartment to regulate its proteolysis and gene expression. J Cell Biol 202:113–127. doi:10.1083/jcb.201211110
Sirac C, Bridoux F, Essig M, Devuyst O, Touchard G, Cogne M (2011) Toward understanding renal Fanconi syndrome: step by step advances through experimental models. Contrib Nephrol 169:247–261. doi:10.1159/000313962
Sousa VH, Fishell G (2010) Sonic hedgehog functions through dynamic changes in temporal competence in the developing forebrain. Curr Opin Genet Dev 20:391–399. doi:10.1016/j.gde.2010.04.008
Storm T, Tranebjaerg L, Frykholm C, Birn H, Verroust PJ, Neveus T, Sundelin B, Hertz JM, Holmstrom G, Ericson K, Christensen EI, Nielsen R (2013) Renal phenotypic investigations of megalin-deficient patients: novel insights into tubular proteinuria and albumin filtration. Nephrol Dial Transplant 28:585–591. doi:10.1093/ndt/gfs462
Storm T, Zeitz C, Cases O, Amsellem S, Verroust PJ, Madsen M, Benoist JF, Passemard S, Lebon S, Jonsson IM, Emma F, Koldso H, Hertz JM, Nielsen R, Christensen EI, Kozyraki R (2013) Detailed investigations of proximal tubular function in Imerslund-Grasbeck syndrome. BMC Med Genet 14:111. doi:10.1186/1471-2350-14-111
Storm T, Heegaard S, Christensen EI, Nielsen R (2014) Megalin-deficiency causes high myopia, retinal pigment epithelium-macromelanosomes and abnormal development of the ciliary body in mice. Cell Tissue Res 358:99–107. doi:10.1007/s00441-014-1919-4
Takeda T, Yamazaki H, Farquhar MG (2003) Identification of an apical sorting determinant in the cytoplasmic tail of megalin. Am J Physiol Cell Physiol 284:C1105–C1113
Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP, van’t Hoff W, Antignac C (1998) A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet 18:319–324. doi:10.1038/ng0498-319
Uhlenhaut NH, Treier M (2008) Transcriptional regulators in kidney disease: gatekeepers of renal homeostasis. Trends Genet 24:361–371. doi:10.1016/j.tig.2008.05.001
Vicinanza M, Di Campli A, Polishchuk E, Santoro M, Di Tullio G, Godi A, Levtchenko E, De Leo MG, Polishchuk R, Sandoval L, Marzolo MP, De Matteis MA (2011) OCRL controls trafficking through early endosomes via PtdIns4,5P(2)-dependent regulation of endosomal actin. EMBO J 30:4970–4985. doi:10.1038/emboj.2011.354
Wallis DE, Muenke M (1999) Molecular mechanisms of holoprosencephaly. Mol Genet Metab 68:126–138. doi:10.1006/mgme.1999.2895
Wallis DE, Roessler E, Hehr U, Nanni L, Wiltshire T, Richieri-Costa A, Gillessen-Kaesbach G, Zackai EH, Rommens J, Muenke M (1999) Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly. Nat Genet 22:196–198
Wang SS, Devuyst O, Courtoy PJ, Wang XT, Wang H, Wang Y, Thakker RV, Guggino S, Guggino WB (2000) Mice lacking renal chloride channel, CLC-5, are a model for Dent’s disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum Mol Genet 9:2937–2945
Wang YP, Dakubo G, Howley P, Campsall KD, Mazarolle CJ, Shiga SA, Lewis PM, McMahon AP, Wallace VA (2002) Development of normal retinal organization depends on Sonic hedgehog signaling from ganglion cells. Nat Neurosci 5:831–832. doi:10.1038/nn911
Watanabe A, Nagai J, Adachi Y, Katsube T, Kitahara Y, Murakami T, Takano M (2004) Targeted prevention of renal accumulation and toxicity of gentamicin by aminoglycoside binding receptor antagonists. J Control Release 95:423–433
Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J (1996) Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci U S A 93:8460–8464
Willnow TE, Christ A, Hammes A (2012) Endocytic receptor-mediated control of morphogen signaling. Development 139:4311–4319. doi:10.1242/dev.084467
Wischnjow A, Sarko D, Janzer M, Kaufman C, Beijer B, Brings S, Haberkorn U, Larbig G, Kubelbeck A, Mier W (2016) Renal targeting: peptide-based drug delivery to proximal tubule cells. Bioconjug Chem 27:1050–1057. doi:10.1021/acs.bioconjchem.6b00057
Wu S, Ren S, Chen H, Chun RF, Gacad MA, Adams JS (2000) Intracellular vitamin D binding proteins: novel facilitators of vitamin D-directed transactivation. Mol Endocrinol 14:1387–1397
Yochem J, Greenwald I (1993) A gene for a low density lipoprotein receptor-related protein in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A 90:4572–4576
Yuseff MI, Farfan P, Bu G, Marzolo MP (2007) A cytoplasmic PPPSP motif determines megalin’s phosphorylation and regulates receptor’s recycling and surface expression. Traffic 8:1215–1230. doi:10.1111/j.1600-0854.2007.00601.x
Zager RA (1996) Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int 49:314–326
Zarbalis K, May SR, Shen Y, Ekker M, Rubenstein JL, Peterson AS (2004) A focused and efficient genetic screening strategy in the mouse: identification of mutations that disrupt cortical development. PLoS Biol 2:E219
Zhang Z, Zheng Q, Han J, Gao G, Liu J, Gong T, Gu Z, Huang Y, Sun X, He Q (2009) The targeting of 14-succinate triptolide-lysozyme conjugate to proximal renal tubular epithelial cells. Biomaterials 30:1372–1381. doi:10.1016/j.biomaterials.2008.11.035
Zhao S, Chen Q, Hung FC, Overbeek PA (2002) BMP signaling is required for development of the ciliary body. Development 129:4435–4442
Zhou D, Liu Y (2016) Renal fibrosis in 2015: understanding the mechanisms of kidney fibrosis. Nat Rev Nephrol 12:68–70. doi:10.1038/nrneph.2015.215
Zoja C, Corna D, Locatelli M, Rottoli D, Pezzotta A, Morigi M, Zanchi C, Buelli S, Guglielmotti A, Perico N, Remuzzi A, Remuzzi G (2015) Effects of MCP-1 inhibition by bindarit therapy in a rat model of polycystic kidney disease. Nephron 129:52–61. doi:10.1159/000369149
Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D (2004) Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem 279:34302–34310. doi:10.1074/jbc.M405608200
Acknowledgements
We are grateful to Julia Klippert for critical reading of the manuscript. Work in the authors’ laboratories was supported by grants SFB665 (to TEW) and CH1838/1-1 (to AC) from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is part of the special issue on Functional Anatomy of the Kidney in Health and Disease in Pflügers Archiv – European Journal of Physiology
Rights and permissions
About this article
Cite this article
Willnow, T.E., Christ, A. Endocytic receptor LRP2/megalin—of holoprosencephaly and renal Fanconi syndrome. Pflugers Arch - Eur J Physiol 469, 907–916 (2017). https://doi.org/10.1007/s00424-017-1992-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-1992-0