Abstract
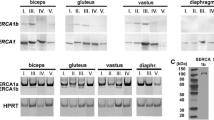
The skeletal muscle ryanodine receptor Ca2+ release channel (RyR1), essential for excitation-contraction (EC) coupling, demonstrates a known developmentally regulated alternative splicing in the ASI region. We now find unexpectedly that the expression of the splice variants is closely related to fiber type in adult human lower limb muscles. We examined the distribution of myosin heavy chain isoforms and ASI splice variants in gluteus minimus, gluteus medius and vastus medialis from patients aged 45 to 85 years. There was a strong positive correlation between ASI(+)RyR1 and the percentage of type 2 fibers in the muscles (r = 0.725), and a correspondingly strong negative correlation between the percentages of ASI(+)RyR1 and percentage of type 1 fibers. When the type 2 fiber data were separated into type 2X and type 2A, the correlation with ASI(+)RyR1 was stronger in type 2X fibers (r = 0.781) than in type 2A fibers (r = 0.461). There was no significant correlation between age and either fiber-type composition or ASI(+)RyR1/ASI(−)RyR1 ratio. The results suggest that the reduced expression of ASI(−)RyR1 during development may reflect a reduction in type 1 fibers during development. Preferential expression of ASI(−) RyR1, having a higher gain of in Ca2+ release during EC coupling than ASI(+)RyR1, may compensate for the reduced terminal cisternae volume, fewer junctional contacts and reduced charge movement in type 1 fibers.







Similar content being viewed by others
References
Agbulut O, Noirez P, Beaumont F, Butler-Browne G (2003) Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell 95:399–406
Andersen JL (2003) Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 13:40–47
Andersen G, Orngreen MC, Preisler N, Colding-Jorgensen E, Clausen T, Duno M, Jeppesen TD, Vissing J (2013) Muscle phenotype in patients with myotonic dystrophy type 1. Muscle Nerve 47:409–415. doi:10.1002/mus.23535
Baylor SM, Hollingworth S (2012) Intracellular calcium movements during excitation-contraction coupling in mammalian slow-twitch and fast-twitch muscle fibers. J Gen Physiol 139:261–272. doi:10.1085/jgp.201210773
Burkholder TJ, Fingado B, Baron S, Lieber RL (1994) Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol 221:177–190. doi:10.1002/jmor.1052210207
Cheng W, Altafaj X, Ronjat M, Coronado R (2005) Interaction between the dihydropyridine receptor Ca2+ channel beta-subunit and ryanodine receptor type 1 strengthens excitation-contraction coupling. Proc Natl Acad Sci U S A 102:19225–19230. doi:10.1073/pnas.0504334102
Cvetko E, Karen P, Erzen I (2012) Myosin heavy chain composition of the human sternocleidomastoid muscle. Ann Anat 194:467–472. doi:10.1016/j.aanat.2012.05.001
Damiani E, Larsson L, Margreth A (1996) Age-related abnormalities in regulation of the ryanodine receptor in rat fast-twitch muscle. Cell Calcium 19:15–27
Dedkov EI, Kostrominova TY, Borisov AB, Carlson BM (2001) Reparative myogenesis in long-term denervated skeletal muscles of adult rats results in a reduction of the satellite cell population. Anat Rec 263:139–154. doi:10.1002/ar.1087
Deschenes MR (2011) Motor unit and neuromuscular junction remodeling with aging. Curr Aging Sci 4:209–220
Dulhunty AF, Gage PW (1983) Asymmetrical charge movement in slow- and fast-twitch mammalian muscle fibres in normal and paraplegic rats. J Physiol 341:213–231
Essen-Gustavsson B, Borges O (1986) Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta Physiol Scand 126:107–114. doi:10.1111/j.1748-1716.1986.tb07793.x
Franzini-Armstrong C, Ferguson DG, Champ C (1988) Discrimination between fast- and slow-twitch fibres of guinea pig skeletal muscle using the relative surface density of junctional transverse tubule membrane. J Muscle Res Cell Motil 9:403–414
Franzini-Armstrong C, Protasi F, Ramesh V (1999) Shape, size, and distribution of Ca2+ release units and couplons in skeletal and cardiac muscles. Biophys J 77:1528–1539. doi:10.1016/S0006-3495(99)77000-1
Futatsugi A, Kuwajima G, Mikoshiba K (1995) Tissue-specific and developmentally regulated alternative splicing in mouse skeletal muscle ryanodine receptor mRNA. Biochem J 305(Pt 2):373–378
Harper PS (2001) Myotonic dystrophy. 3rd ed. / with a chapter on molecular and cell biology by J. David Brook and Emma Newman. edn. W.B. Saunders, London
Johnson MA, Polgar J, Weightman D, Appleton D (1973) Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci 18:111–129
Kimura T, Lueck JD, Harvey PJ, Pace SM, Ikemoto N, Casarotto MG, Dirksen RT, Dulhunty AF (2009) Alternative splicing of RyR1 alters the efficacy of skeletal EC coupling. Cell Calcium 45:264–274. doi:10.1016/j.ceca.2008.11.005
Kimura T, Nakamori M, Lueck JD, Pouliquin P, Aoike F, Fujimura H, Dirksen RT, Takahashi MP, Dulhunty AF, Sakoda S (2005) Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in myotonic dystrophy type 1. Hum Mol Genet 14:2189–2200. doi:10.1093/hmg/ddi223
Kimura T, Pace SM, Wei L, Beard NA, Dirksen RT, Dulhunty AF (2007) A variably spliced region in the type 1 ryanodine receptor may participate in an inter-domain interaction. Biochem J 401:317–324. doi:10.1042/BJ20060686
Lamboley CR, Murphy RM, McKenna MJ, Lamb GD (2013) Endogenous and maximal sarcoplasmic reticulum calcium content and calsequestrin expression in type I and type II human skeletal muscle fibres. J Physiol 591:6053–6068. doi:10.1113/jphysiol.2013.265900
Larsson L, Sjodin B, Karlsson J (1978) Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand 103:31–39. doi:10.1111/j.1748-1716.1978.tb06187.x
Lexell J (1995) Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50 Spec No:11–16
Lexell J, Taylor CC, Sjostrom M (1988) What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84:275–294
Mizunoya W, Wakamatsu J, Tatsumi R, Ikeuchi Y (2008) Protocol for high-resolution separation of rodent myosin heavy chain isoforms in a mini-gel electrophoresis system. Anal Biochem 377:111–113. doi:10.1016/j.ab.2008.02.021
O’Connell K, Gannon J, Doran P, Ohlendieck K (2008) Reduced expression of sarcalumenin and related Ca2+−regulatory proteins in aged rat skeletal muscle. Exp Gerontol 43:958–961. doi:10.1016/j.exger.2008.07.006
Porter MM, Vandervoort AA, Lexell J (1995) Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports 5:129–142
Renganathan M, Messi ML, Delbono O (1997) Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J Membr Biol 157:247–253
Renganathan M, Messi ML, Delbono O (1998) Overexpression of IGF-1 exclusively in skeletal muscle prevents age-related decline in the number of dihydropyridine receptors. J Biol Chem 273:28845–28851
Rhee HS, Lucas CA, Hoh JF (2004) Fiber types in rat laryngeal muscles and their transformations after denervation and reinnervation. J Histochem Cytochem 52:581–590
Rivero JL, Talmadge RJ, Edgerton VR (1998) Fibre size and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. J Muscle Res Cell Motil 19:733–742
Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91:1447–1531. doi:10.1152/physrev.00031.2010
Soukup T, Diallo M (2015) Proportions of myosin heavy chain mRNAs, protein isoforms and fiber types in the slow and fast skeletal muscles are maintained after alterations of thyroid status in rats. Physiol Res 64:111–118
Talmadge RJ, Roy RR (1993) Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol 75:2337–2340
Xu L, Wang Y, Yamaguchi N, Pasek DA, Meissner G (2008) Single channel properties of heterotetrameric mutant RyR1 ion channels linked to core myopathies. J Biol Chem 283:6321–6329. doi:10.1074/jbc.M707353200
Yan Z, Bai XC, Yan C, Wu J, Li Z, Xie T, Peng W, Yin CC, Li X, Scheres SH, Shi Y, Yan N (2015) Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 517:50–55. doi:10.1038/nature14063
Yu F, Hedstrom M, Cristea A, Dalen N, Larsson L (2007) Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol (Oxf) 190:229–241. doi:10.1111/j.1748-1716.2007.01699.x
Zorzato F, Sacchetto R, Margreth A (1994) Identification of two ryanodine receptor transcripts in neonatal, slow-, and fast-twitch rabbit skeletal muscles. Biochem Biophys Res Commun 203:1725–1730. doi:10.1006/bbrc.1994.2385
Acknowledgments
The authors are grateful to Suzy Pace and to Joan Stivala for assistance with the collection of the human tissue and to NA Beard for helpful comment on the manuscript. The work was supported by grants from the National Health and Medical Research Council APP1020589 and APP1002589 as well as an Australian Postgraduate Award and a John Curtin School of Medical Research supplementary scholarship to HW.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 485 kb)
Rights and permissions
About this article
Cite this article
Willemse, H., Theodoratos, A., Smith, P.N. et al. Unexpected dependence of RyR1 splice variant expression in human lower limb muscles on fiber-type composition. Pflugers Arch - Eur J Physiol 468, 269–278 (2016). https://doi.org/10.1007/s00424-015-1738-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1738-9