Abstract
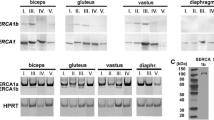
The neonatal isoform of the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA1b) is formed by developmental splicing and expressed fully only in developing muscle. As a major Ca2+ pump in myotubes, SERCA1b must be detected in excitation contraction coupling or in store-operated calcium entry. The available pan SERCA1 antibodies also recognise SERCA1b but these are more frequently used to detect SERCA1a, the adult muscle-specific isoform characteristically expressed in fast fibres of skeletal muscle. In such applications, the pan SERCA1 antibodies are frequently claimed to be SERCA1a antibodies without proving it. Realistically, such an antibody cannot be made since it should recognise a single glycine at the C-terminal, the only part of SERCA1a that is different from SERCA1b. The false interpretation of the antibody specificity created inconsistence in the literature and led to false conclusions attributing features only to SERCA1a although those at least are also shared by SERCA1b. In contrast, a SERCA1b antibody has been made against the eight amino acid peptide tail that replaces the glycine of SERCA1a at the C-terminal. Therefore, the expression of SERCA1b can be specifically demonstrated, unlike that of SERCA1a, in various stages and conditions of skeletal muscle. This review argues against misbeliefs related to the distinction, expressions and functions of the two muscle-specific SERCA1 isoforms.


Similar content being viewed by others
References
Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M (2007) Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol 43:215–22
Brandl CJ, deLeon S, Martin DR, MacLennan DH (1987) Adult forms of the Ca2+ ATPase of sarcoplasmic reticulum. Expression in developing skeletal muscle. J Biol Chem 262:3768–3774
Brandl CJ, Green NM, Korczak B, MacLennan DH (1986) Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell 44:597–607
Chami M, Gozuacik D, Lagorce D, Brini M, Falson P, Peaucellier G, Pinton P, Lecoeur H, Gougeon ML, le Maire M, Rizzuto R, Bréchot C, Paterlini-Bréchot P (2001) SERCA1 truncated proteins unable to pump calcium reduce the endoplasmic reticulum calcium concentration and induce apoptosis. J Cell Biol 153:1301–14
Collet C, Ma J (2004) Calcium-dependent facilitation and graded deactivation of store-operated calcium entry in fetal skeletal muscle. Biophys J 87:268–275
Fajardo VA, Bombardier E, Vigna C, Devji T, Bloemberg D, Gamu D, Gramolini AO, Quadrilatero J, Tupling AR (2013) Co-expression of SERCA isoforms, phospholamban and sarcolipin in human muscles. PLoS ONE 8:e84304. doi:10.1371/journal.pone.0084304
Goonasekera SA, Lam CK, Millay DP, Sargent MA, Hajjar RJ, Kranias EG, Molkentin JD (2011) Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J Clin Invest 121:1044–52. doi:10.1172/JCI43844
Guglielmi V, Vattemi G, Gualandi F, Voermans NC, Marini M, Scotton C, Pegoraro E, Oosterhof A, Kósa M, Zádor E, Valente EM, De Grandis D, Neri M, Codemo V, Novelli A, van Kuppevelt TH, Dallapiccola B, van Engelen BG, Ferlini A, Tomelleri G (2013) SERCA1 protein expression in muscle of patients with Brody disease and Brody syndrome and in cultured human muscle fibers. Mol Genet Metab 110:162–9. doi:10.1016/j.ymgme.2013.07.015
Guglielmi V, Voermans NC, Gualandi F, Van Engelen BG, Ferlini A, Tomelleri G, Vattemi G (2013) Fourty-four years of Brody disease: it is time to review. J Genet Syndr Gene Ther 4:2. doi:10.4172/2157-7412.1000181
Kimura T, Nakamori M, Lueck JD, Pouliquin P, Aoike F, Fujimura H, Dirksen RT, Takahashi MP, Dulhunty AF, Sakoda S (2005) Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2 + -ATPase in myotonic dystrophy type 1. Hum Mol Genet 14:2189–2200
Kósa M, Zádor E (2013) Transfection efficiency along the regenerating soleus muscle of the rat. Mol Biotechnol 54:220–227. doi:10.1007/s12033-012-9555-2
Launikonis BS, Murphy RM, Edwards JN (2010) Toward the roles of store-operated Ca2+ entry in skeletal muscle. Pflugers Arch 460:813–23. doi:10.1007/s00424-010-0856-7
Lee KJ, Hyun C, Woo JS, Park CS, do Kim H, Lee EH (2014) Stromal interaction molecule 1 (STIM1) regulates sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 1a (SERCA1a) in skeletal muscle. Pflugers Arch 466:987–1001
Manjarrés IM, Rodríguez-García A, Alonso MT, García-Sancho J (2010) The sarco/endoplasmic reticulum Ca(2+) ATPase (SERCA) is the third element in capacitative calcium entry. Cell Calcium 47:412–8. doi:10.1016/j.ceca.2010.03.001
Maruyama K, MacLennan DH (1988) Mutation of aspartic acid-351, lysine-352, and lysine-515 alters the Ca2+ transport activity of the Ca2 + -ATPase expressed in COS-1 cells. Proc Natl Acad Sci U S A 85:3314–3318
Mendler L, Szakonyi G, Zádor E, Görbe A, Dux L, Wuytack F (1998) Expression of sarcoplasmic/endoplasmic reticulum Ca2+ ATPases in the rat extensor digitorum longus (EDL) muscle regenerating from notexin-induced necrosis. J Muscle Res Cell Motil 19:777–785
Michalak M, Opas M (2009) Endoplasmic and sarcoplasmic reticulum in the heart. Trends Cell Biol 19:253–259. doi:10.1016/j.tcb.2009.03.006
Odermatt A, Taschner PE, Khanna VK, Busch HF, Karpati G, Jablecki CK, Breuning MH, MacLennan DH (1996) Mutations in the gene-encoding SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+ ATPase, are associated with Brody disease. Nat Genet 14:191–4
Pan Y, Zvaritch E, Tupling AR, Rice WJ, de Leon S, Rudnicki M, McKerlie C, Banwell BL, MacLennan DH (2003) Targeted disruption of the ATP2A1 gene encoding the sarco(endo)plasmic reticulum Ca2+ ATPase isoform 1 (SERCA1) impairs diaphragm function and is lethal in neonatal mice. J Biol Chem 278:13367–13375
Periasamy M, Kalyanasundaram A (2007) SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve 35:430–42
Porter GJ, Makuck R, Rivkees S (2002) Reduction in intracellular calcium levels inhibits myoblast differentiation. J Biol Chem 277:28942–28947
Putney JJ (1986) A model for receptor-regulated calcium entry. Cell Calcium 7:1–12
Putney JW (2013) Alternative forms of the store-operated calcium entry mediators, STIM1 and Orai1. Curr Top Membr 71:109–23. doi:10.1016/B978-0-12-407870-3.00005-6
Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91:1447–1531. doi:10.1152/physrev.00031.2010
Schneider JS, Shanmugam M, Gonzalez JP, Lopez H, Gordan R, Fraidenraich D, Babu GJ (2013) Increased sarcolipin expression and decreased sarco(endo)plasmic reticulum Ca2+ uptake in skeletal muscles of mouse models of Duchenne muscular dystrophy. J Muscle Res Cell Motil 34:349–356. doi:10.1007/s10974-013-9350-0
Seth M, Li T, Graham V, Burch J, Finch E, Stiber JA, Rosenberg PB (2012) Dynamic regulation of sarcoplasmic reticulum Ca(2+) stores by stromal interaction molecule 1 and sarcolipin during muscle differentiation. Dev Dyn 241:639–647. doi:10.1002/dvdy.23760
Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P (2008) STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol 10:688–697. doi:10.1038/ncb1731
Stiber JA, Rosenberg PB (2011) The role of store-operated calcium influx in skeletal muscle signaling. Cell Calcium 49:341–349. doi:10.1016/j.ceca.2010.11.012
Szabó A, Wuytack F, Zádor E (2008) The effect of passive movement on denervated soleus highlights a differential nerve control on SERCA and MyHC isoforms. J Histochem Cytochem 56:1013–22. doi:10.1369/jhc.2008.951632
Talmadge RJ, Roy RR, Chalmers GR, Edgerton VR (1996) MHC and sarcoplasmic reticulum protein isoforms in functionally overloaded cat plantaris muscle fibers. J Appl Physiol 80:1296–1303
Vangheluwe P, Sepulveda MR, Missiaen L, Raeymaekers L, Wuytack F, Vanoevelen J (2009) Intracellular Ca2+- and Mn2+-transport ATPases. Chem Rev 109:4733–4759. doi:10.1021/cr900013m
Vihola A, Bassez G, Meola G, Zhang S, Haapasalo H, Paetau A, Mancinelli E, Rouche A, Hogrel JY, Laforêt P, Maisonobe T, Pellissier JF, Krahe R, Eymard B, Udd B (2003) Histopathological differences of myotonic dystrophy type 1 (DM1) and PROMM/DM2. Neurology 60:1854–1857
Vihola A, Sirito M, Bachinski LL, Raheem O, Screen M, Suominen T, Krahe R, Udd B (2013) Altered expression and splicing of Ca (2+) metabolism genes in myotonic dystrophies DM1 and DM2. Neuropathol Appl Neurobiol 39:390–405. doi:10.1111/j.1365-2990.2012.01289.x
Zádor E, Dux L, Wuytack F (1999) Prolonged passive stretch of rat soleus muscle provokes an increase in the mRNA levels of the muscle regulatory factors distributed along the entire length of the fibers. J Muscle Res Cell Motil 20:395–402
Zádor E, Mendler L, Ver Heyen M, Dux L, Wuytack F (1996) Changes in mRNA levels of the sarcoplasmic/endoplasmic-reticulum Ca(2+)-ATPase isoforms in the rat soleus muscle regenerating from notexin-induced necrosis. Biochem J 320:107–113
Zádor E, Owsianik G, Wuytack F (2011) Silencing SERCA1b in a few fibers stimulates growth in the entire regenerating soleus muscle. Histochem Cell Biol 135:11–20. doi:10.1007/s00418-010-0766-y
Zádor E, Szakonyi G, Rácz G, Mendler L, Ver Heyen M, Lebacq J, Dux L, Wuytack F (1998) Expression of the sarco/endoplasmic reticulum Ca(2+)-transport ATPase protein isoforms during regeneration from notexin-induced necrosis of rat soleus muscle. Acta Histochem 100:355–369
Zádor E, Vangheluwe P, Wuytack F (2007) The expression of the neonatal sarcoplasmic reticulum Ca2+ pump (SERCA1b) hints to a role in muscle growth and development. Cell Calcium 41:379–88
Zubrzycka-Gaarn E, MacDonald G, Phillips L, Jorgensen AO, MacLennan DH (1984) Monoclonal antibodies to the Ca2+ + Mg2+ -dependent ATPase of sarcoplasmic reticulum identify polymorphic forms of the enzyme and indicate the presence in the enzyme of a classical high-affinity Ca2+ binding site. J Bioenerg Biomembr 16:441–464
Acknowledgements
The study of SERCA1b was supported by grants TéT B-20/04 in Hungary and BIL 02/18 in Belgium. Thank you to prof. F. Wuytack for antibodies and advices. M.K. was supported by TÁMOP 4.2.4 and A/2-11-1-2012-0001 'National Excellence Program' grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zádor, E., Kósa, M. The neonatal sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA1b): a neglected pump in scope. Pflugers Arch - Eur J Physiol 467, 1395–1401 (2015). https://doi.org/10.1007/s00424-014-1671-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1671-3