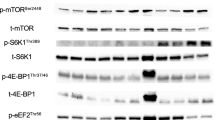
Abstract
Knowledge on the effects of divergent exercise on ostensibly protein degradation pathways may be valuable for counteracting muscle wasting and for understanding muscle remodelling. This study examined mRNA and/or protein levels of molecular markers of the ubiquitin proteasome pathway (UPP), including FBXO32 (atrogin-1), MURF-1, FBXO40, FOXO1 and FOXO3. Protein substrates of atrogin-1—including EIF3F, MYOG and MYOD1—and of MURF-1—including PKM and MHC—were also measured. Subjects completed 10 weeks of endurance training (ET) or resistance training (RT) followed by a single-bout of endurance exercise (EE) or resistance exercise (RE). Following training, atrogin-1, FBXO40, FOXO1 and FOXO3 mRNA increased independently of exercise mode, whereas MURF-1 mRNA and FOXO3 protein increased following ET only. No change in other target proteins occurred post-training. In the trained state, single-bout EE, but not RE, increased atrogin-1, MURF-1, FBXO40, FOXO1, FOXO3 mRNA and FOXO3 protein. In contrast to EE, FBXO40 mRNA and protein decreased following single-bout RE. MURF-1 and FOXO1 protein levels as well as the protein substrates of atrogin-1 and MURF-1 were unchanged following training and single-bout exercise. This study demonstrates that the intracellular signals elicited by ET and RT result in an upregulation of UPP molecular markers, with a greater increase following ET. However, in the trained state, the expression levels of UPP molecular markers are increased following single-bout EE, but are less responsive to single-bout RE. This suggests that adaptations following endurance exercise training are more reliant on protein UPP degradation processes than adaptations following resistance exercise training.






Similar content being viewed by others
References
Andersen LB (1995) A maximal cycle exercise protocol to predict maximal oxygen uptake. Scand J Med Sci Sports 5(3):143–146. doi:10.1111/j.1600-0838.1995.tb00027.x
Andersen JL, Aagaard P (2000) Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve 23(7):1095–1104
Andersen LL, Tufekovic G, Zebis MK, Crameri RM, Verlaan G, Kjaer M, Suetta C, Magnusson P, Aagaard P (2005) The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metabolism 54(2):151–156. doi:10.1016/j.metabol.2004.07.012
Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR (1995) Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol 268(3 Pt 1):E514–E520
Biolo G, Williams BD, Fleming RY, Wolfe RR (1999) Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes 48(5):949–957. doi:10.2337/diabetes.48.5.949
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294(5547):1704–1708. doi:10.1126/science.1065874
Borgenvik M, Apro W, Blomstrand E (2012) Intake of branched-chain amino acids influences the levels of MAFbx mRNA and MuRF-1 total protein in resting and exercising human muscle. Am J Physiol Endocrinol Metab 302(5):E510–E521. doi:10.1152/ajpendo.00353.2011
Brooke MH, Kaiser KK (1970) Muscle fiber types: how many and what kind? Arch Neurol 23(4):369–379. doi:10.1001/archneur.1970.00480280083010
Brzycki M (1993) Strength testing: predicting a one-rep max from a reps-to-fatigue. J Phys Educ Recreat Dance 64(1):88–90
Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG (2010) Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc 42(10):1843–1852. doi:10.1249/MSS.0b013e3181d964e4
Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR (1990) Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol 259(4 Pt 1):E470–E476
Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S (2001) Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol 306(4):717–726. doi:10.1006/jmbi.2001.4448
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159. doi:10.1006/abio.1987.9999
Churchley EG, Coffey VG, Pedersen DJ, Shield A, Carey KA, Cameron-Smith D, Hawley JA (2007) Influence of preexercise muscle glycogen content on transcriptional activity of metabolic and myogenic genes in well-trained humans. J Appl Physiol 102(4):1604–1611. doi:10.1152/japplphysiol.01260.2006
Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ (2007) The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab 6(5):376–385. doi:10.1016/j.cmet.2007.09.009
Coffey VG, Shield A, Canny BJ, Carey KA, Cameron-Smith D, Hawley JA (2006) Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes. Am J Physiol Endocrinol Metab 290(5):E849–E855. doi:10.1152/ajpendo.00299.2005
Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL (2009) During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol 185(6):1083–1095. doi:10.1083/jcb.200901052
Cunha TF, Moreira JB, Paixao NA, Campos JC, Monteiro AW, Bacurau AV, Bueno CR Jr, Ferreira JC, Brum PC (2012) Aerobic exercise training upregulates skeletal muscle calpain and ubiquitin-proteasome systems in healthy mice. J Appl Physiol 112(11):1839–1846. doi:10.1152/japplphysiol.00346.2011
Dalbo VJ, Roberts MD, Hassell S, Kerksick CM (2013) Effects of pre-exercise feeding on serum hormone concentrations and biomarkers of myostatin and ubiquitin proteasome pathway activity. Eur J Nutr 52(2):477–487. doi:10.1007/s00394-012-0349-x
Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M (2008) Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol 104(2):371–378. doi:10.1152/japplphysiol.00873.2007
Farup J, Kjolhede T, Sorensen H, Dalgas U, Moller AB, Vestergaard PF, Ringgaard S, Bojsen-Moller J, Vissing K (2012) Muscle morphological and strength adaptations to endurance vs. resistance training. J Strength Cond Res 26(2):398–407. doi:10.1519/JSC.0b013e318225a26f
Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, Rasmussen BB (2010) Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol 299(2):R533–R540. doi:10.1152/ajpregu.00077.2010
Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A 98(25):14440–14445. doi:10.1073/pnas.251541198
Green H, Goreham C, Ouyang J, Ball-Burnett M, Ranney D (1999) Regulation of fiber size, oxidative potential, and capillarization in human muscle by resistance exercise. Am J Physiol 276:R591–R596
Hirner S, Krohne C, Schuster A, Hoffmann S, Witt S, Erber R, Sticht C, Gasch A, Labeit S, Labeit D (2008) MuRF1-dependent regulation of systemic carbohydrate metabolism as revealed from transgenic mouse studies. J Mol Biol 379(4):666–677. doi:10.1016/j.jmb.2008.03.049
Holloszy JO, Coyle EF (1984) Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56:831–838
Hood DA, Terjung RL (1987) Leucine metabolism in perfused rat skeletal muscle during contractions. Am J Physiol 253(6 Pt 1):E636–E647
Hulmi JJ, Kovanen V, Selanne H, Kraemer WJ, Hakkinen K, Mero AA (2009) Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids 37(2):297–308. doi:10.1007/s00726-008-0150-6
Jogo M, Shiraishi S, Tamura TA (2009) Identification of MAFbx as a myogenin-engaged F-box protein in SCF ubiquitin ligase. FEBS Lett 583(17):2715–2719. doi:10.1016/j.febslet.2009.07.033
Kedar V, McDonough H, Arya R, Li HH, Rockman HA, Patterson C (2004) Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci U S A 101(52):18135–18140. doi:10.1073/pnas.0404341102
Kim PL, Staron RS, Phillips SM (2005) Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol 568(Pt 1):283–290. doi:10.1113/jphysiol.2005.093708
Kostek MC, Chen YW, Cuthbertson DJ, Shi R, Fedele MJ, Esser KA, Rennie MJ (2007) Gene expression responses over 24 h to lengthening and shortening contractions in human muscle: major changes in CSRP3, MUSTN1, SIX1, and FBXO32. Physiol Genomics 31(1):42–52. doi:10.1152/physiolgenomics.00151.2006
Lagirand-Cantaloube J, Offner N, Csibi A, Leibovitch MP, Batonnet-Pichon S, Tintignac LA, Segura CT, Leibovitch SA (2008) The initiation factor eIF3-f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J 27(8):1266–1276. doi:10.1038/emboj.2008.52
Lamon S, Wallace MA, Stefanetti RJ, Rahbek SK, Vendelbo MH, Russell AP, Vissing K (2013) Regulation of the STARS signaling pathway in response to endurance and resistance exercise and training. Pflugers Arch - Eur J Physiol. doi:10.1007/s00424-013-1265-5
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18(1):39–51. doi:10.1096/fj.03-0610com
Lecker SH, Goldberg AL, Mitch WE (2006) Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17(7):1807–1819. doi:10.1681/ASN.2006010083
Leger B, Cartoni R, Praz M, Lamon S, Deriaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP (2006) Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol 576(Pt 3):923–933. doi:10.1113/jphysiol.2006.116715
Louis E, Raue U, Yang Y, Jemiolo B, Trappe S (2007) Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103(5):1744–1751. doi:10.1152/japplphysiol.00679.2007
Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA (2005) Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J 19(11):1498–1500. doi:10.1096/fj.04-3149fje
Mascher H, Andersson H, Nilsson PA, Ekblom B, Blomstrand E (2007) Changes in signalling pathways regulating protein synthesis in human muscle in the recovery period after endurance exercise. Acta Physiol (Oxf) 191(1):67–75. doi:10.1111/j.1748-1716.2007.01712.x
Mascher H, Tannerstedt J, Brink-Elfegoun T, Ekblom B, Gustafsson T, Blomstrand E (2008) Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am J Physiol Endocrinol Metab 294(1):E43–E51. doi:10.1152/ajpendo.00504.2007
Nedergaard A, Vissing K, Overgaard K, Kjaer M, Schjerling P (2007) Expression patterns of atrogenic and ubiquitin proteasome component genes with exercise: effect of different loading patterns and repeated exercise bouts. J Appl Physiol 103(5):1513–1522. doi:10.1152/japplphysiol.01445.2006
Pasiakos SM, McClung HL, McClung JP, Urso ML, Pikosky MA, Cloutier GJ, Fielding RA, Young AJ (2010) Molecular responses to moderate endurance exercise in skeletal muscle. Int J Sport Nutr Exerc Metab 20(4):282–290
Peserico A, Chiacchiera F, Grossi V, Matrone A, Latorre D, Simonatto M, Fusella A, Ryall JG, Finley LW, Haigis MC, Villani G, Puri PL, Sartorelli V, Simone C (2013) A novel AMPK-dependent FoxO3A-SIRT3 intramitochondrial complex sensing glucose levels. Cell Mol Life Sci: CMLS 70(11):2015–2029. doi:10.1007/s00018-012-1244-6
Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR (1997) Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol 273(1 Pt 1):E99–E107. doi:10.1097/00005768-199705001-01319
Phillips SM, Tipton KD, Ferrando AA, Wolfe RR (1999) Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol 276(1 Pt 1):E118–E124
Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S (2007) Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci 62(12):1407–1412
Rauramaa R, Kuusela P, Hietanen E (1980) Adipose, muscle and lung tissue lipoprotein lipase activities in young streptozotocin treated rats. Horm Metab Res Hormon Stoffwechs Hormon Metab 12(11):591–595. doi:10.1055/s-2007-999207
Reitelseder S, Agergaard J, Doessing S, Helmark IC, Schjerling P, van Hall G, Kjaer M, Holm L (2013) Positive muscle protein net balance and differential regulation of atrogene expression after resistance exercise and milk protein supplementation. Eur J Nutr. doi:10.1007/s00394-013-0530-x
Russell AP (2010) Molecular regulation of skeletal muscle mass. Clin Exp Pharmacol Physiol 37(3):378–384. doi:10.1111/j.1440-1681.2009.05265.x
Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL (2007) Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J 21(1):140–155. doi:10.1096/fj.06-6604com
Sale D, MacDougall D (1981) Specificity in strength training: a review for the coach and athlete. Can J Appl Sport Sci J Can Sci Appl Sport 6(2):87–92
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117(3):399–412. doi:10.1016/S0092-8674(04)00400-3
Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, Mammucari C, Meskers CG, Pallafacchina G, Paoli A, Pion D, Roceri M, Romanello V, Serrano AL, Toniolo L, Larsson L, Maier AB, Munoz-Canoves P, Musaro A, Pende M, Reggiani C, Rizzuto R, Schiaffino S (2013) Signalling pathways regulating muscle mass in ageing skeletal muscle. The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 14(3):303–323. doi:10.1007/s10522-013-9432-9
Shi J, Luo L, Eash J, Ibebunjo C, Glass DJ (2011) The SCF-Fbxo40 complex induces IRS1 ubiquitination in skeletal muscle, limiting IGF1 signaling. Dev Cell 21(5):835–847. doi:10.1016/j.devcel.2011.09.011
Stefanetti RJ, Lamon S, Rahbek SK, Farup J, Zacharewicz E, Wallace MA, Vendelbo MH, Russell AP, Vissing K (2014) Influence of divergent exercise contraction mode and whey protein supplementation on atrogin-1, MuRF1 and FOXO1/3A in human skeletal muscle. J Appl Physiol. doi:10.1152/japplphysiol.00136.2013
Stefanetti RJ, Zacharewicz E, Della Gatta P, Garnham A, Russell AP, Lamon S (2014) Ageing has no effect on the regulation of the ubiquitin proteasome-related genes and proteins following resistance exercise. Front Physiol 5:30. doi:10.3389/fphys.2014.00030
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14(3):395–403. doi:10.1016/S1097-2765(04)00211-4
Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA (2005) Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem 280(4):2847–2856. doi:10.1074/jbc.M411346200
Toth MJ, Tchernof A (2006) Effect of age on skeletal muscle myofibrillar mRNA abundance: relationship to myosin heavy chain protein synthesis rate. Exp Gerontol 41(11):1195–1200. doi:10.1016/j.exger.2006.08.005
van Wessel T, de Haan A, van der Laarse WJ, Jaspers RT (2010) The muscle fiber type-fiber size paradox: hypertrophy or oxidative metabolism? Eur J Appl Physiol 110(4):665–694. doi:10.1007/s00421-010-1545-0
Vissing K, Brink M, Lonbro S, Sorensen H, Overgaard K, Danborg K, Mortensen J, Elstrom O, Rosenhoj N, Ringgaard S, Andersen JL, Aagaard P (2008) Muscle adaptations to plyometric vs. resistance training in untrained young men. J Strength Cond Res 22(6):1799–1810. doi:10.1519/JSC.0b013e318185f673
Vissing K, McGee SL, Roepstorff C, Schjerling P, Hargreaves M, Kiens B (2008) Effect of sex differences on human MEF2 regulation during endurance exercise. Am J Physiol Endocrinol Metab 294(2):E408–E415. doi:10.1152/ajpendo.00403.2007
Vissing K, McGee SL, Farup J, Kjolhede T, Vendelbo MH, Jessen N (2011) Differentiated mTOR but not AMPK signaling after strength vs endurance exercise in training-accustomed individuals. Scand J Med Sci Sports. doi:10.1111/j.1600-0838.2011.01395.x
Wakshlag JJ, Kallfelz FA, Barr SC, Ordway G, Haley NJ, Flaherty CE, Kelley RL, Altom EK, Lepine AJ, Davenport GM (2002) Effects of exercise on canine skeletal muscle proteolysis: an investigation of the ubiquitin-proteasome pathway and other metabolic markers. Vet Ther: Res Appl Vet Med 3(3):215–225
Yan Z, Lira VA, Greene NP (2012) Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev 40(3):159–164. doi:10.1097/JES.0b013e3182575599
Yang Y, Jemiolo B, Trappe S (2006) Proteolytic mRNA expression in response to acute resistance exercise in human single skeletal muscle fibers. J Appl Physiol 101(5):1442–1450. doi:10.1152/japplphysiol.00438.2006
Acknowledgments
The sarcomeric myosin (MF 20) hybridoma antibody developed by Donald A. Fischman was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. We thank the subjects for their participation in the project. Jean Farup, Tue Kjølhede, Andreas Buch Møller and Poul Vestergaard are thanked for the assistance with training and testing of the subjects. Séverine Lamon was supported by an Alfred Deakin Postdoctoral fellowship from Deakin University. Kristian Vissing was supported by the Novo Nordisk Foundation. The study complied with the current laws of Region Midtjylland, Denmark.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOCX 42 kb)
Rights and permissions
About this article
Cite this article
Stefanetti, R.J., Lamon, S., Wallace, M. et al. Regulation of ubiquitin proteasome pathway molecular markers in response to endurance and resistance exercise and training. Pflugers Arch - Eur J Physiol 467, 1523–1537 (2015). https://doi.org/10.1007/s00424-014-1587-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1587-y