Abstract
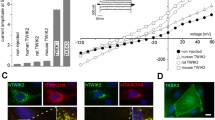
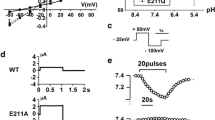
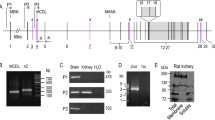
We have identified a novel splice variant of the human and rat two-pore domain potassium (K2P) channel TREK-1. The splice variant TREK-1e results from skipping of exon 5, which causes a frame shift in exon 6. The frame shift produces a novel C-terminal amino acid sequence and a premature termination of translation, which leads to a loss of transmembrane domains M3 and M4 and of the second pore domain. RT-PCR experiments revealed a preferential expression of TREK-1e in kidney, adrenal gland, and amygdala. TREK-1e was nonfunctional when expressed in Xenopus oocytes. However, both the surface expression and the current density of full-length TREK-1 were reduced by co-expression of TREK-1e. Live cell imaging in COS-7 cells transfected with GFP-tagged TREK-1e showed that this splice variant was retained in the endoplasmic reticulum (ER). Attachment of the C-terminus of TREK-1e to two different reporter proteins (Kir2.1 and CD8) led to a strong reduction in the surface expression of these fusion proteins. Progressive truncation of the C-terminus of TREK-1e in these reporter constructs revealed a critical region (amino acids 198 to 205) responsible for the intracellular retention. Mutagenesis experiments indicated that amino acids I204 and W205 are key residues mediating the ER retention of TREK-1e. Our results suggest that the TREK-1e splice variant may interfere with the vesicular traffic of full-length TREK-1 channels from the ER to the plasma membrane. Thus, TREK-1e might modulate the copy number of functional TREK-1 channels at the cell surface, providing a novel mechanism for fine tuning of TREK-1 currents.







Similar content being viewed by others
References
Bittner S, Ruck T, Schuhmann MK, Herrmann AM, Maati HM, Bobak N, Gobel K, Langhauser F, Stegner D, Ehling P, Borsotto M, Pape HC, Nieswandt B, Kleinschnitz C, Heurteaux C, Galla HJ, Budde T, Wiendl H, Meuth SG (2013) Endothelial TWIK-related potassium channel-1 (TREK1) regulates immune-cell trafficking into the CNS. Nat Med 19:1161–1165. doi:10.1038/nm.3303
Bockenhauer D, Zilberberg N, Goldstein SA (2001) KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat Neurosci 4:486–491. doi:10.1038/87434
Brohawn SG, del Marmol J, MacKinnon R (2012) Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science 335:436–441. doi:10.1126/science.1213808
Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M (1996) Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J 15:6854–6862, PMID: 9003761
Franks NP, Honore E (2004) The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol Sci 25:601–608. doi:10.1016/j.tips.2004.09.003
Gu W, Schlichthörl G, Hirsch JR, Engels H, Karschin C, Karschin A, Derst C, Steinlein OK, Daut J (2002) Expression pattern and functional characteristics of two novel splice variants of the two-pore-domain potassium channel TREK-2. J Physiol 539:657–668. doi:10.1113/jphysiol.2001.013432
Han J, Kang D, Kim D (2003) Functional properties of four splice variants of a human pancreatic tandem-pore K+ channel, TALK-1. Am J Physiol Cell Physiol 285:C529–C538. doi:10.1152/ajpcell.00601.2002
Kim D (2005) Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des 11:2717–2736. doi:10.2174/1381612054546824
Kim Y, Bang H, Kim D (1999) TBAK-1 and TASK-1, two-pore K+ channel subunits: kinetic properties and expression in rat heart. Am J Physiol 277:H1669–H1678, PMID: 10564119
Ma D, Nakata T, Zhang G, Hoshi T, Li M, Shikano S (2007) Differential trafficking of carboxyl isoforms of Ca2+-gated (Slo1) potassium channels. FEBS Lett 581:1000–1008. doi:10.1016/j.febslet.2007.01.077
Ma D, Zerangue N, Lin YF, Collins A, Yu M, Jan YN, Jan LY (2001) Role of ER export signals in controlling surface potassium channel numbers. Science 291:316–319. doi:10.1126/science.291.5502.316
Miller AN, Long SB (2012) Crystal structure of the human two-pore domain potassium channel K2P1. Science 335:432–436. doi:10.1126/science.1213274
Ozaita A, Vega-Saenz de Miera E (2002) Cloning of two transcripts, HKT4.1a and HKT4.1b, from the human two-pore K+ channel gene KCNK4. Chromosomal localization, tissue distribution and functional expression. Brain Res Mol Brain Res 102:18–27
Patel AJ, Maingret F, Magnone V, Fosset M, Lazdunski M, Honore E (2000) TWIK-2, an inactivating 2P domain K+ channel. J Biol Chem 275:28722–28730. doi:10.1074/jbc.M003755200
Renigunta V, Yuan H, Zuzarte M, Rinné S, Koch A, Wischmeyer E, Schlichthörl G, Gao Y, Karschin A, Jacob R, Schwappach B, Daut J, Preisig-Müller R (2006) The retention factor p11 confers an endoplasmic reticulum-localization signal to the potassium channel TASK-1. Traffic 7:168–181. doi:10.1111/j.1600-0854.2005.00375.x
Schutze MP, Peterson PA, Jackson MR (1994) An N-terminal double-arginine motif maintains type II membrane proteins in the endoplasmic reticulum. EMBO J 13:1696–1705
Simkin D, Cavanaugh EJ, Kim D (2008) Control of the single channel conductance of K2P10.1 (TREK-2) by the amino-terminus: role of alternative translation initiation. J Physiol 586:5651–5663. doi:10.1113/jphysiol.2008.161927
Staudacher K, Baldea I, Kisselbach J, Staudacher I, Rahm AK, Schweizer PA, Becker R, Katus HA, Thomas D (2011) Alternative splicing determines mRNA translation initiation and function of human K2P10.1 K+ channels. J Physiol 589:3709–3720. doi:10.1113/jphysiol.2011.210666
Thomas D, Plant LD, Wilkens CM, McCrossan ZA, Goldstein SA (2008) Alternative translation initiation in rat brain yields K2P2.1 potassium channels permeable to sodium. Neuron 58:859–870. doi:10.1016/j.neuron.2008.04.016
Veale EL, Rees KA, Mathie A, Trapp S (2010) Dominant negative effects of a non-conducting TREK1 splice variant expressed in brain. J Biol Chem 285:29295–29304. doi:10.1074/jbc.M110.108423
Wu YY, Singer CA, Buxton IL (2012) Variants of stretch-activated two-pore potassium channel TREK-1 associated with preterm labor in humans. Biol Reprod 87:96. doi:10.1095/biolreprod.112.099499
Xian Tao L, Dyachenko V, Zuzarte M, Putzke C, Preisig-Müller R, Isenberg G, Daut J (2006) The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc Res 69:86–97. doi:10.1016/j.cardiores.2005.08.018
Zerangue N, Schwappach B, Jan YN, Jan LY (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron 22:537–548
Zerangue N, Malan MJ, Fried SR, Dazin PF, Jan YN, Jan LY, Schwappach B (2001) Analysis of endoplasmic reticulum trafficking signals by combinatorial screening in mammalian cells. Proc Natl Acad Sci U S A 98:2431–2436. doi:10.1073/pnas.05163019898/5/2431
Zuzarte M, Rinné S, Schlichthörl G, Schubert A, Daut J, Preisig-Müller R (2007) A di-acidic sequence motif enhances the surface expression of the potassium channel TASK-3. Traffic 8:1093–1100. doi:10.1111/j.1600-0854.2007.00593.x
Zuzarte M, Heusser K, Renigunta V, Schlichthörl G, Rinné S, Wischmeyer E, Daut J, Schwappach B, Preisig-Müller R (2009) Intracellular traffic of the K+ channels TASK-1 and TASK-3: role of N- and C-terminal sorting signals and interaction with 14-3-3 proteins. J Physiol 587:929–952. doi:10.1113/jphysiol.2008.164756
Acknowledgments
This work was supported by grants of the Deutsche Forschungsgemeinschaft SFB 593, TP A4 to J.D. and 1482-3/2 to N.D., the P.E. Kempkes Foundation (to S.R. and R.P.M.), and the Anneliese Pohl Stiftung to S.R.. We thank Caroline Rolfes and Thorsten Steinfeldt for the isolation of porcine adrenal gland. We are grateful to Andrea Schubert for excellent technical support.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rinné, S., Renigunta, V., Schlichthörl, G. et al. A splice variant of the two-pore domain potassium channel TREK-1 with only one pore domain reduces the surface expression of full-length TREK-1 channels. Pflugers Arch - Eur J Physiol 466, 1559–1570 (2014). https://doi.org/10.1007/s00424-013-1384-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-013-1384-z