Abstract
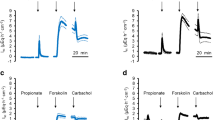
ATP-sensitive K+ (KATP) channels couple the metabolic state of a cell to its electrical activity. They consist of a hetero-octameric complex with pore-forming Kir6.x (Kir6.1, Kir6.2) and regulatory sulfonylurea receptor (SUR) subunits. Functional data indicate that KATP channels contribute to epithelial K+ currents at colonic epithelia. However, their molecular identity and their properties are largely unknown. Therefore, changes in short-circuit current (I sc) induced by the KATP channel opener pinacidil (5 10−4 mol l−1) were measured in Ussing chambers under control conditions and in the presence of different blockers of KATP channels. The channel subunits expressed by the colonic epithelium were identified by immunohistochemistry and by RT-PCR. The K+ channel opener, when administered at the serosal side, induced an increase in I sc consistent with the induction of transepithelial Cl− secretion after activation of basolateral K+ channels, whereas mucosal administration of pinacidil resulted in a negative I sc. The increase in I sc evoked by serosal pinacidil was inhibited by serosal administration of glibenclamide (5 10−4 mol l−1) and gliclazide (10−6 mol l−1), but was resistant even against a high concentration (10−2 mol l−1) of tolbutamide. In contrast, none of these inhibitors (administered at the mucosal side) reduced significantly the negative I sc induced by mucosal pinacidil. Instead, pinacidil inhibited Cl− currents across apical Cl− channels in basolaterally depolarized epithelia indicating that the negative I sc induced by mucosal pinacidil is due to a transient inhibition of Cl− secretion. In mRNA prepared from isolated colonic crypts, messenger RNA for both pore-forming subunits, Kir6.1 and Kir6.2, and two regulatory subunits (SUR1and SUR2B) was found. Expression within the colonic epithelium was confirmed for these subunits by immunohistochemistry. In consequence, KATP channels are present in the basolateral membrane of the colonic epithelium; their exact subunit composition, however, has still to be revealed.









Similar content being viewed by others
References
Amoroso S, Schmid-Antomarchi H, Fosset M, Lazdunski M (1990) Glucose, sulphonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science 247:852–854
Ashcroft FM, Harrison DE, Ashcroft SJ (1984) Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 312:446–448
Ashcroft FM, Gribble FM (1999) ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia 42:903–919
Ashcroft FM, Gribble FM (2000) New windows on the mechanism of action of KATP channel openers. Trends Pharmacol Sci 21:439–445
Babenko AP, Aguilar-Bryan L, Bryan J (1998) A view of SUR/KIR6.x, KATP channels. Annu Rev Physiol 60:667–687
Blondeau N, Plamondon H, Richelme C, Heurteaux C, Lazdunski M (2000) KATP channel openers, adenosine agonists and epileptic preconditioning are stress signals inducing hippocampal neuroprotection. Neuroscience 100:465–474
Cao K, Tang G, Hu D, Wang R (2002) Molecular basis of ATP-sensitive K+ channels in rat vascular smooth muscles. Biochem Biophys Res Commun 296:463–469
Chutkow WA, Makielski JC, Nelson DJ, Burant CF, Fan Z (1999) Alternative splicing of SUR2 exon 17 regulates nucleotide sensitivity of the ATP-sensitive potassium channels. J Biol Chem 274:13656–13665
Cook DL, Hales CN (1984) Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 311:271–273
Cook NS, Quast U (1990) Potassium channels. Structure, classification, function and therapeutic potential. In: Cook NS (ed) Potassium channel pharmacology. Ellis Horwood, New York, pp 181–255
Edwards G, Weston AH (1993) The pharmacology of ATP-sensitive K+ channels. Annu Rev Pharmacol Toxicol 33:597–637
Greger R (2000) Role of CFTR in the colon. Annu Rev Physiol 62:467–491
Gribble FM, Tucker SJ, Seino S, Ashcroft FM (1998) Tissue specificity of sulfonylureas: studies on cloned cardiac and beta-cell KATP channels. Diabetes 47:1412–1418
Hennig B, Diener M (2009) Actions of hydrogen sulfide on ion transport across rat distal colon. Brit J Pharmacol 158:1263–1275
Hennig B, Schultheiss G, Kunzelmann K, Diener M (2008) Ca2+-induced Cl− efflux at rat distal colonic epithelium. J Membrane Biol 221:61–72
Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J (1995) Reconstitution of IKATP: an inward rectifier subunit plus the sulphonylurea receptor. Science 270:1166–1170
Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S (1996) A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron 16:1011–1017
Inoue I, Nagase H, Kishi K, Higuti T (1991) ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature 352:244–247
Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y (1996) A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem 271:24321–24324
Jöns T, Wittschieber D, Beyer A, Meier C, Brune A, Thomzig A, Ahnert-Hilger G, Veh RW (2006) K+-ATP-channel-related protein complexes: potential transducers in the regulation of epithelial tight junction permeability. J Cell Sci 119:3087–3097
Liu M, Seino S, Kirchgessner A (1999) Identification and characterization of glucoresponsive neurons in the enteric nervous system. J Neurosci 19:10305–10317
Maguire D, MacNamara B, Cuffe JE, Winter D, Doolan CM, Urbach V, O' Sullivan GC, Harvey BJ (1999) Rapid responses to aldosterone in human distal colon. Steroids 64:51–63
Meddings JB (1997) Review article: intestinal permeability in Crohn’s disease. Aliment Pharmacol Ther 11(Suppl 3):47–53
Noma A (1983) ATP-regulated K+ channels in cardiac muscle. Nature 305:147–148
Olaison G, Sjödahl R, Tagesson C (1990) Abnormal intestinal permeability in Crohn’s disease. A possible pathogenic factor. Scand J Gastroenterol 25:321–8
Ploug KB, Edvinsson L, Olesen J, Jansen-Olesen I (2006) Pharmacological and molecular comparison of KATP channels in rat basilar and middle cerebral arteries. Eur J Pharmacol 553:254–262
Pouokam E, Diener M (2011) Mechanims of actions of hydrogen sulfide at rat distal colonic epithelium. Brit J Pharmacol 162:392–404
Schultheiss G, Kocks SL, Diener M (2002) Methods for the study of ionic currents and Ca2+-signals in isolated colonic crypts. Biol Proced Online 3:70–78 (http://www.biologicalprocedures.com/)
Seino S, Miki T (2003) Physiological and pathophysiological roles of ATP-sensitive K+ channels. Progr Biophys Mol Biol 81:133–176
Sheppard DN, Welsh MJ (1992) Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride channels. J Gen Physiol 100:573–591
Söderholm JD, Olaison G, Peterson KH, Franzén LE, Lindmark T, Wirén M, Tagesson C, Sjödahl R (2002) Augmented increase in tight junction permeability by luminal stimuli in the non-inflamed ileum of Crohn’s disease. Gut 50:307–313
Spruce AE, Standen NB, Standfield PR (1985) Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature 316:736–738
Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT (1989) Hyperpolarizing vasodilatators activate ATP-sensitive K+ channels in arterial smooth muscle. Science 245:177–180
Strabel D, Diener M (1995) Evidence against direct activation of chloride secretion by carbachol in the rat distal colon. Eur J Pharmacol 274:181–191
Tilmann M, Kunzelmann K, Fröbe U, Cabantchik I, Lang HJ, Englert HC, Greger R (1991) Different types of blockers of the intermediate-conductance outwardly rectifying chloride channel in epithelia. Pflügers Arch Eur J Physiol 418:556–563
Wallace JL, Vong L, McKnight W, Dicay M, Gary RM (2009) Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology 137:569–578
Wulfsen I, Hauber HP, Schiemann D, Bauer CK, Schwarz JR (2000) Expression of mRNA for voltage-dependent and inward-rectifying K channels in GH3/B6 cells and rat pituitary. J Neuroendocrinol 12:263–272
Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y (1997) Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol 499:715–720
Zhou M, He HJ, Hirano M, Sekiguchi M, Tanaka O, Kawahara K, Abe H (2010) Localization of ATP-sensitive K+ channel subunits in rat submandibular gland. J Histochem Cytochem 58:499–507
Zhou M, He HJ, Suzuki R, Liu KX, Tanaka O, Sekiguchi M, Itoh H, Kawahara K, Abe H (2007) Localization of sulfonylurea receptor subunits, SUR2A and SUR2B, in rat heart. J Histochem Cytochem 55:795–804
Zhou M, Tanaka O, Sekiguchi M, He HJ, Yasuoka Y, Itoh H, Kawahara K, Abe H (2005) ATP-sensitive K+-channel subunits on the mitochondria and endoplasmic reticulum of rat cardiomyocytes. J Histochem Cytochem 53:1491–1500
Acknowledgments
Supported by Deutsche Forschungsgemeinschaft, grant Di 388/11-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pouokam, E., Bader, S., Brück, B. et al. ATP-sensitive K+ channels in rat colonic epithelium. Pflugers Arch - Eur J Physiol 465, 865–877 (2013). https://doi.org/10.1007/s00424-012-1207-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-012-1207-7