Abstract
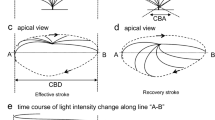
Airway ciliary beat activity (CBA) plays a pivotal role in protecting the body by removing mucus and pathogens from the respiratory tract. Since CBA is complicated and cannot be characterized by merely frequency, we recorded CBA using laser confocal line scanning and defined six parameters for describing CBA. The values of these parameters were all above 0 when measured in beating ciliated cells from mouse tracheae. We subsequently used 10 μM adenosine-5′-triphosphate (ATP) to stimulate ciliated cells and simultaneously recorded intracellular Ca2+ levels and CBA. We found that intracellular Ca2+ levels first increased, followed by an increase in CBA. Among the six parameters, frequency, amplitude, and integrated area significantly increased, whereas rise time, decay time, and full duration at half maximum markedly decreased. The results suggest that these six parameters are appropriate for assessing CBA and that increased intracellular Ca2+ levels might enhance CBA. We next used our established methods to observe changes in mechanically stimulated cilia tips. We found that mechanical stimulation-induced changes in both intracellular Ca2+ levels and CBA were not only similar to those induced by ATP, but were also blocked by treatment with a Ca2+ chelator, BAPTA-AM, (10 μM) for 10 min. Moreover, while the same blockage was observed under Ca2+-free conditions, addition of 2 mM Ca2+ into the chamber restored increases in both intracellular Ca2+ levels and CBA. Taken together, we have provided a novel method for real-time measurement and complete analysis of CBA as well as demonstrated that mechanical stimulation of cilia tips resulted in Ca2+ influx that led to increased intracellular Ca2+ levels, which in turn triggered CBA enhancement.







Similar content being viewed by others
References
Dalhamn T (1955) A method for determination in vivo of the rate of ciliary beat and mucous flow in the trachea. Acta Physiol Scand 33:1–5
Dalhamn T (1960) The determination in vivo of the rate of ciliary beat in the trachea. Acta Physiol Scand 49:242–250
Doyle RT, Moninger T, Debavalya N, Hsu WH (2006) Use of confocal linescan to document ciliary beat frequency. J Microsc 223:159–164
Evans JH, Sanderson MJ (1999) Intracellular calcium oscillations regulate ciliary beat frequency of airway epithelial cells. Cell Calcium 26:103–110
Geary CA, Davis CW, Paradiso AM, Boucher RC (1995) Role of CNP in human airways: cGMP-mediated stimulation of ciliary beat frequency. Am J Physiol 268:L1021–L1028
Kakuta Y, Kanno T, Sasaki H, Takishima T (1985) Effect of Ca2+ on the ciliary beat frequency of skinned dog tracheal epithelium. Respir Physiol 60:9–19
Korngreen A, Priel Z (1996) Purinergic stimulation of rabbit ciliated airway epithelia: control by multiple calcium sources. J Physiol 497(Pt 1):53–66
Lansley AB, Sanderson MJ (1999) Regulation of airway ciliary activity by Ca2+: simultaneous measurement of beat frequency and intracellular Ca2+. Biophys J 77:629–638
Lorenzo IM, Liedtke W, Sanderson MJ, Valverde MA (2008) TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci USA 105:12611–12616
Ma W, Silberberg SD, Priel Z (2002) Distinct axonemal processes underlie spontaneous and stimulated airway ciliary activity. J Gen Physiol 120:875–885
Ma W, Korngreen A, Uzlaner N, Priel Z, Silberberg SD (1999) Extracellular sodium regulates airway ciliary motility by inhibiting a P2X receptor. Nature 400:894–897
Moore WD, Engelmann TW (1869) On ciliary motion. J Anat Physiol 3:420–435
Nlend MC, Bookman RJ, Conner GE, Salathe M (2002) Regulator of G-protein signaling protein 2 modulates purinergic calcium and ciliary beat frequency responses in airway epithelia. Am J Respir Cell Mol Biol 27:436–445
Porter ME, Sale WS (2000) The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol 151:F37–F42
Salathe M, Bookman RJ (1999) Mode of Ca2+ action on ciliary beat frequency in single ovine airway epithelial cells. J Physiol 520(Pt 3):851–865
Sanderson MJ, Dirksen ER (1986) Mechanosensitivity of cultured ciliated cells from the mammalian respiratory tract: implications for the regulation of mucociliary transport. Proc Natl Acad Sci USA 83:7302–7306
Sanderson MJ, Lansley AB, Dirksen ER (1992) Regulation of ciliary beat frequency in respiratory tract cells. Chest 101:69S–71S
Verdugo P (1980) Ca2+-dependent hormonal stimulation of ciliary activity. Nature 283:764–765
Wirschell M, Yamamoto R, Alford L, Gokhale A, Gaillard A et al (2011) Regulation of ciliary motility: conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme. Arch Biochem Biophys 510:93–100
Zhang L, Sanderson MJ (2003) Oscillations in ciliary beat frequency and intracellular calcium concentration in rabbit tracheal epithelial cells induced by ATP. J Physiol 546:733–749
Zhang L, Sanderson MJ (2003) The role of cGMP in the regulation of rabbit airway ciliary beat frequency. J Physiol 551:765–776
Acknowledgments
This work was supported by the 973 Research Program (2011CB809100) and the National Natural Science Foundation of China (31140087 and 30971514 to Q-HL).
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wen-Er Li and Weiwei Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, WE., Chen, W., Ma, YF. et al. Methods to measure and analyze ciliary beat activity: Ca2+ influx-mediated cilia mechanosensitivity. Pflugers Arch - Eur J Physiol 464, 671–680 (2012). https://doi.org/10.1007/s00424-012-1164-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-012-1164-1