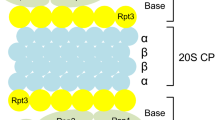
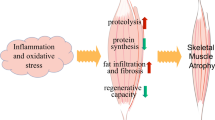
Abstract
Skeletal muscle atrophy occurs in many chronic diseases and disuse conditions. Its severity reduces patient recovery, independence and quality of life. The discovery of two muscle-specific E3 ubiquitin ligases, MAFbx/atrogin-1 and Muscle RING Finger-1 (MuRF1), promoted an expectation of these molecules as targets for therapeutic development. While numerous studies have determined the conditions in which MAFbx/atrogin-1 and MuRF1 mRNA levels are regulated, few studies have investigated their functional role in skeletal muscle. Recently, studies identifying new target substrates for MAFbx/atrogin-1 and MuRF1, outside of their response to the initiation of muscle atrophy, suggest that there is more to these proteins than previously appreciated. This review will highlight our present knowledge of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy, the impact of potential therapeutics and their known regulators and substrates. Finally, we will comment on new approaches that may expand our knowledge of these two molecules in their control of skeletal muscle function.


Similar content being viewed by others
References
Allen DL, Bandstra ER, Harrison BC, Thorng S, Stodieck LS, Kostenuik PJ, Morony S, Lacey DL, Hammond TG, Leinwand LL, Argraves WS, Bateman TA, Barth JL (2009) Effects of spaceflight on murine skeletal muscle gene expression. J Appl Physiol 106:582–595
Asp ML, Tian M, Wendel AA, Belury MA (2010) Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. Int J Cancer 126:756–763
Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE (1996) The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin–proteasome pathway. J Clin Invest 97:1447–1453
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Bonetto A, Penna F, Minero VG, Reffo P, Bonelli G, Baccino FM, Costelli P (2009) Deacetylase inhibitors modulate the myostatin/follistatin axis without improving cachexia in tumor-bearing mice. Curr Cancer Drug Targets 9:608–616
Bossola M, Muscaritoli M, Costelli P, Grieco G, Bonelli G, Pacelli F, Rossi Fanelli F, Doglietto GB, Baccino FM (2003) Increased muscle proteasome activity correlates with disease severity in gastric cancer patients. Ann Surg 237:384–389
Brack AS, Rando TA (2007) Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3:226–237
Cai D, Frantz JD, Tawa NE Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE (2004) IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119:285–298
Caron AZ, Drouin G, Desrosiers J, Trensz F, Grenier G (2009) A novel hindlimb immobilization procedure for studying skeletal muscle atrophy and recovery in mouse. J Appl Physiol 106:2049–2059
Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang M-L, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S (2001) Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol 306:717–726
Chen YW, Gregory CM, Scarborough MT, Shi R, Walter GA, Vandenborne K (2007) Transcriptional pathways associated with skeletal muscle disuse atrophy in humans. Physiol Genomics 31:510–520
Chen Y, Cao L, Ye J, Zhu D (2009) Upregulation of myostatin gene expression in streptozotocin-induced type 1 diabetes mice is attenuated by insulin. Biochem Biophys Res Commun 388:112–116
Chicheportiche Y, Chicheportiche R, Sizing I, Thompson J, Benjamin CB, Ambrose C, Dayer JM (2002) Proinflammatory activity of TWEAK on human dermal fibroblasts and synoviocytes: blocking and enhancing effects of anti-TWEAK monoclonal antibodies. Arthritis Res 4:126–133
Chikanza IC, Roux-Lombard P, Dayer JM, Panayi GS (1995) Dysregulation of the in vivo production of interleukin-1 receptor antagonist in patients with rheumatoid arthritis. Pathogenetic implications. Arthritis Rheum 38:642–648
Choi H, Selpides P, Nowell M, Rourke BC (2009) Functional overload in ground squirrel plantaris muscle fails to induce myosin isoform shifts. Am J Physiol Regul Integr Comp Physiol 297:R578–R586
Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B (2006) Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat Tibialis Anterior muscle. Mech Ageing Dev 127:794–801
Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL (2009) During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol 185:1083–1095
Costelli P, Muscaritoli M, Bossola M, Penna F, Reffo P, Bonetto A, Busquets S, Bonelli G, Lopez-Soriano FJ, Doglietto GB, Argiles JM, Baccino FM, Rossi Fanelli F (2006) IGF-1 is downregulated in experimental cancer cachexia. Am J Physiol Regul Integr Comp Physiol 291:R674–683
Csibi A, Tintignac LA, Leibovitch MP, Leibovitch SA (2008) eIF3-f function in skeletal muscles: to stand at the crossroads of atrophy and hypertrophy. Cell Cycle 7:1698–1701
Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ (2005) Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19:422–424
Dehoux M, Van Beneden R, Pasko N, Lause P, Verniers J, Underwood L, Ketelslegers JM, Thissen JP (2004) Role of the insulin-like growth factor I decline in the induction of atrogin-1/MAFbx during fasting and diabetes. Endocrinology 145:4806–4812
Doucet M, Russell AP, Leger B, Debigare R, Joanisse DR, Caron MA, Leblanc P, Maltais F (2007) Muscle atrophy and hypertrophy signalling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 176:261–269
Dudley GA, Castro MJ, Rogers S, Apple DF Jr (1999) A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol 80:394–396
Edstrom E, Altun M, Hagglund M, Ulfhake B (2006) Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J Gerontol A Biol Sci Med Sci 61:663–674
Fang CH, James HJ, Ogle C, Fischer JE, Hasselgren PO (1995) Influence of burn injury on protein metabolism in different types of skeletal muscle and the role of glucocorticoids. J Am Coll Surg 180:33–42
Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren PO (2006) Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol 290:R1589–R1597
Fielitz J, Kim MS, Shelton JM, Latif S, Spencer JA, Glass DJ, Richardson JA, Bassel-Duby R, Olson EN (2007) Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J Clin Invest 117:2486–2495
Foletta VC, Prior MJ, Stupka N, Carey K, Segal DH, Jones S, Swinton C, Martin S, Cameron-Smith D, Walder KR (2009) NDRG2, a novel regulator of myoblast proliferation, is regulated by anabolic and catabolic factors. J Physiol 587:1619–1634
Frost RA, Nystrom GJ, Jefferson LS, Lang CH (2007) Hormone, cytokine, and nutritional regulation of sepsis-induced increases in atrogin-1 and MuRF1 in skeletal muscle. Am J Physiol Endocrinol Metab 292:E501–E512
Goldberg AL (1969) Protein turnover in skeletal muscle. II. Effects of denervation and cortisone on protein catabolism in skeletal muscle. J Biol Chem 244:3223–3229
Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98:14440–14445
Gonzalez-Cadavid NF, Taylor WE, Yarasheski K, Sinha-Hikim I, Ma K, Ezzat S, Shen R, Lalani R, Asa S, Mamita M, Nair G, Arver S, Bhasin S (1998) Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc Natl Acad Sci USA 95:14938–14943
Graham KA, Reaich D, Channon SM, Downie S, Goodship TH (1997) Correction of acidosis in hemodialysis decreases whole-body protein degradation. J Am Soc Nephrol 8:632–637
Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A (2005) Ghrelin receptor agonist GHRP-2 prevents arthritis-induced increase in E3 ubiquitin-ligating enzymes MuRF1 and MAFbx gene expression in skeletal muscle. Am J Physiol Endocrinol Metab 289:E1007–E1014
Granado M, Martin AI, Priego T, Lopez-Calderon A, Villanua MA (2006) Tumour necrosis factor blockade did not prevent the increase of muscular muscle RING finger-1 and muscle atrophy F-box in arthritic rats. J Endocrinol 191:319–326
Granado M, Martin AI, Villanua MA, Lopez-Calderon A (2007) Experimental arthritis inhibits the insulin-like growth factor-I axis and induces muscle wasting through cyclooxygenase-2 activation. Am J Physiol Endocrinol Metab 292:E1656–E1665
Gustafsson T, Osterlund T, Flanagan JN, von Walden F, Trappe TA, Linnehan RM, Tesch PA (2010) Effects of 3 days unloading on molecular regulators of muscle size in humans. J Appl Physiol 109:721–727
Haddad F, Adams GR, Bodell PW, Baldwin KM (2006) Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J Appl Physiol 100:433–441
Hansen MJ, Gualano RC, Bozinovski S, Vlahos R, Anderson GP (2006) Therapeutic prospects to treat skeletal muscle wasting in COPD (chronic obstructive lung disease). Pharmacol Ther 109:162–172
Harber MP, Crane JD, Dickinson JM, Jemiolo B, Raue U, Trappe TA, Trappe SW (2009) Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles. Am J Physiol Regul Integr Comp Physiol 296:R708–R714
Hirner S, Krohne C, Schuster A, Hoffmann S, Witt S, Erber R, Sticht C, Gasch A, Labeit S, Labeit D (2008) MuRF1-dependent regulation of systemic carbohydrate metabolism as revealed from transgenic mouse studies. J Mol Biol 379:666–677
Hong-Brown LQ, Brown CR, Lang CH (2004) Indinavir impairs protein synthesis and phosphorylations of MAPKs in mouse C2C12 myocytes. Am J Physiol Cell Physiol 287:C1482–C1492
Hong-Brown LQ, Pruznak AM, Frost RA, Vary TC, Lang CH (2005) Indinavir alters regulators of protein anabolism and catabolism in skeletal muscle. Am J Physiol Endocrinol Metab 289:E382–E390
Joazeiro CA, Weissman AM (2000) RING finger proteins: mediators of ubiquitin ligase activity. Cell 102:549–552
Jogo M, Shiraishi S, Tamura TA (2009) Identification of MAFbx as a myogenin-engaged F-box protein in SCF ubiquitin ligase. FEBS Lett 583:2715–2719
Jones SW, Hill RJ, Krasney PA, O’Conner B, Peirce N, Greenhaff PL (2004) Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J 18:1025–1027
Kline WO, Panaro FJ, Yang H, Bodine SC (2007) Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J Appl Physiol 102:740–747
Krawiec BJ, Frost RA, Vary TC, Jefferson LS, Lang CH (2005) Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am J Physiol Endocrinol Metab 289:E969–E980
Lagirand-Cantaloube J, Offner N, Csibi A, Leibovitch MP, Batonnet-Pichon S, Tintignac LA, Segura CT, Leibovitch SA (2008) The initiation factor eIF3-f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J 27:1266–1276
Lagirand-Cantaloube J, Cornille K, Csibi A, Batonnet-Pichon S, Leibovitch MP, Leibovitch SA (2009) Inhibition of atrogin-1/MAFbx mediated MyoD proteolysis prevents skeletal muscle atrophy in vivo. PLoS ONE 4:e4973
Lang CH, Frost RA, Vary TC (2007) Skeletal muscle protein synthesis and degradation exhibit sexual dimorphism after chronic alcohol consumption but not acute intoxication. Am J Physiol Endocrinol Metab 292:E1497–E1506
Larsen AE, Tunstall RJ, Carey KA, Nicholas G, Kambadur R, Crowe TC, Cameron-Smith D (2006) Actions of short-term fasting on human skeletal muscle myogenic and atrogenic gene expression. Ann Nutr Metab 50:476–481
Lecker SH, Solomon V, Mitch WE, Goldberg AL (1999) Muscle protein breakdown and the critical role of the ubiquitin–proteasome pathway in normal and disease states. J Nutr 129:227S–237S
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18:39–51
Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE (2004) Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin–proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol 15:1537–1545
Leger B, Vergani L, Soraru G, Hespel P, Derave W, Gobelet C, D’Ascenzio C, Angelini C, Russell AP (2006) Human skeletal muscle atrophy in amyotrophic lateral sclerosis reveals a reduction in Akt and an increase in atrogin-1. FASEB J 20:583–585
Leger B, Derave W, De Bock K, Hespel P, Russell AP (2008) Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res 11:163B–175B
Leger B, Senese R, Al-Khodairy AW, Deriaz O, Gobelet C, Giacobino JP, Russell AP (2009) Atrogin-1, MuRF1, and FoXO, as well as phosphorylated GSK-3beta and 4E-BP1 are reduced in skeletal muscle of chronic spinal cord-injured patients. Muscle Nerve 40:69–78
Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, Patterson C (2004) Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest 114:1058–1071
Lopez-Menduina M, Martin AI, Castillero E, Villanua MA, Lopez-Calderon A (2010) Systemic IGF-I administration attenuates the inhibitory effect of chronic arthritis on gastrocnemius mass and decreases atrogin-1 and IGFBP-3. Am J Physiol Regul Integr Comp Physiol 299:R541–R551
Louis E, Raue U, Yang Y, Jemiolo B, Trappe S (2007) Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103:1744–1751
Lynch GS (2001) Therapies for improving muscle function in neuromuscular disorders. Exerc Sport Sci Rev 29:141–148
Mantovani G, Madeddu C (2009) Cancer cachexia: medical management. Support Care Cancer 18:1–9
Marinovic AC, Zheng B, Mitch WE, Price SR (2007) Tissue-specific regulation of ubiquitin (UbC) transcription by glucocorticoids: in vivo and in vitro analyses. Am J Physiol Ren Physiol 292:F660–F666
Mastrocola R, Reffo P, Penna F, Tomasinelli CE, Boccuzzi G, Baccino FM, Aragno M, Costelli P (2008) Muscle wasting in diabetic and in tumor-bearing rats: role of oxidative stress. Free Radic Biol Med 44:584–593
Menconi M, Gonnella P, Petkova V, Lecker S, Hasselgren PO (2008) Dexamethasone and corticosterone induce similar, but not identical, muscle wasting responses in cultured L6 and C2C12 myotubes. J Cell Biochem 105:353–364
Mitch WE (2007) Malnutrition is an unusual cause of decreased muscle mass in chronic kidney disease. J Ren Nutr 17:66–69
Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR (1999) Evaluation of signals activating ubiquitin–proteasome proteolysis in a model of muscle wasting. Am J Physiol 276:C1132–C1138
Mitch WE, Du J, Bailey JL, Price SR (1999) Mechanisms causing muscle proteolysis in uremia: the influence of insulin and cytokines. Miner Electrolyte Metab 25:216–219
Moresi V, Williams AH, Meadows E, Flynn JM, Potthoff MJ, McAnally J, Shelton JM, Backs J, Klein WH, Richardson JA, Bassel-Duby R, Olson EN (2010) Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 143:35–45
Moylan JS, Smith JD, Chambers MA, McLoughlin TJ, Reid MB (2008) TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am J Physiol Cell Physiol 295:C986–C993
Murphy KT, Lynch GS (2009) Update on emerging drugs for cancer cachexia. Expert Opin Emerg Drugs 14:619–632
Muscaritoli M, Bossola M, Aversa Z, Bellantone R, Rossi Fanelli F (2006) Prevention and treatment of cancer cachexia: new insights into an old problem. Eur J Cancer 42:31–41
Naderi J, Bernreuther C, Grabinski N, Putman CT, Henkel B, Bell G, Glatzel M, Sultan KR (2009) Plasminogen activator inhibitor type 1 up-regulation is associated with skeletal muscle atrophy and associated fibrosis. Am J Pathol 175:763–771
Nair KS, Woolf PD, Welle SL, Matthews DE (1987) Leucine, glucose, and energy metabolism after 3 days of fasting in healthy human subjects. Am J Clin Nutr 46:557–562
Nakao R, Hirasaka K, Goto J, Ishidoh K, Yamada C, Ohno A, Okumura Y, Nonaka I, Yasutomo K, Baldwin KM, Kominami E, Higashibata A, Nagano K, Tanaka K, Yasui N, Mills EM, Takeda S, Nikawa T (2009) Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Mol Cell Biol 29:4798–4811
Nikawa T, Ishidoh K, Hirasaka K, Ishihara I, Ikemoto M, Kano M, Kominami E, Nonaka I, Ogawa T, Adams GR, Baldwin KM, Yasui N, Kishi K, Takeda S (2004) Skeletal muscle gene expression in space-flown rats. FASEB J 18:522–524
Nordquist J, Hoglund AS, Norman H, Tang X, Dworkin B, Larsson L (2007) Transcription factors in muscle atrophy caused by blocked neuromuscular transmission and muscle unloading in rats. Mol Med 13:461–470
Nystrom G, Pruznak A, Huber D, Frost RA, Lang CH (2009) Local insulin-like growth factor I prevents sepsis-induced muscle atrophy. Metabolism 58:787–797
Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics 1:376–386
Otis JS, Guidot DM (2009) Procysteine stimulates expression of key anabolic factors and reduces plantaris atrophy in alcohol-fed rats. Alcohol Clin Exp Res 33:1450–1459
Otis JS, Ashikhmin YI, Brown LA, Guidot DM (2008) Effect of HIV-1-related protein expression on cardiac and skeletal muscles from transgenic rats. AIDS Res Ther 5:8
Penna F, Costamagna D, Fanzani A, Bonelli G, Baccino FM, Costelli P (2010) Muscle wasting and impaired myogenesis in tumor bearing mice are prevented by ERK inhibition. PLoS ONE 5:e13604
Pickering WP, Price SR, Bircher G, Marinovic AC, Mitch WE, Walls J (2002) Nutrition in CAPD: serum bicarbonate and the ubiquitin–proteasome system in muscle. Kidney Int 61:1286–1292
Plant PJ, Brooks D, Faughnan M, Bayley T, Bain J, Singer L, Correa J, Pearce D, Binnie M, Batt J (2010) Cellular markers of muscle atrophy in chronic obstructive pulmonary disease (COPD). Am J Respir Cell Mol Biol 42:461–471
Poylin V, Fareed MU, O’Neal P, Alamdari N, Reilly N, Menconi M, Hasselgren PO (2008) The NF-kappaB inhibitor curcumin blocks sepsis-induced muscle proteolysis. Mediat Inflamm 2008:317851
Pratt JM, Petty J, Riba-Garcia I, Robertson DH, Gaskell SJ, Oliver SG, Beynon RJ (2002) Dynamics of protein turnover, a missing dimension in proteomics. Mol Cell Proteomics 1:579–591
Preedy VR, Ohlendieck K, Adachi J, Koll M, Sneddon A, Hunter R, Rajendram R, Mantle D, Peters TJ (2003) The importance of alcohol-induced muscle disease. J Muscle Res Cell Motil 24:55–63
Pruznak AM, Hong-Brown L, Lantry R, She P, Frost RA, Vary TC, Lang CH (2008) Skeletal and cardiac myopathy in HIV-1 transgenic rats. Am J Physiol Endocrinol Metab 295:E964–E973
Rajan V, Mitch WE (2008) Ubiquitin, proteasomes and proteolytic mechanisms activated by kidney disease. Biochim Biophys Acta 1782:795–799
Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S (2007) Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci 62:1407–1412
Reaich D, Channon SM, Scrimgeour CM, Daley SE, Wilkinson R, Goodship TH (1993) Correction of acidosis in humans with CRF decreases protein degradation and amino acid oxidation. Am J Physiol 265:E230–E235
Rudich A, Konrad D, Torok D, Ben-Romano R, Huang C, Niu W, Garg RR, Wijesekara N, Germinario RJ, Bilan PJ, Klip A (2003) Indinavir uncovers different contributions of GLUT4 and GLUT1 towards glucose uptake in muscle and fat cells and tissues. Diabetologia 46:649–658
Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL (2004) IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287:E591–E601
Sakuma K, Watanabe K, Hotta N, Koike T, Ishida K, Katayama K, Akima H (2009) The adaptive responses in several mediators linked with hypertrophy and atrophy of skeletal muscle after lower limb unloading in humans. Acta Physiol Oxf 197:151–159
Salanova M, Schiffl G, Püttmann B, Schoser BG, Blottner D (2008) Molecular biomarkers monitoring human skeletal muscle fibres and microvasculature following long-term bed rest with and without countermeasures. J Anat 212:306–318
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412
Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM (2006) PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA 103:16260–16265
Saxne T, Palladino MA Jr, Heinegard D, Talal N, Wollheim FA (1988) Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum 31:1041–1045
Servais S, Letexier D, Favier R, Duchamp C, Desplanches D (2007) Prevention of unloading-induced atrophy by vitamin E supplementation: links between oxidative stress and soleus muscle proteolysis? Free Radic Biol Med 42:627–635
Siddiqui RA, Hassan S, Harvey KA, Rasool T, Das T, Mukerji P, Demichele S (2009) Attenuation of proteolysis and muscle wasting by curcumin c3 complex in MAC16 colon tumour-bearing mice. Br J Nutr 102:967–975
Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, Lecker S, Taegtmeyer H, Goldberg AL, Walsh K (2005) The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem 280:20814–20823
Solomon V, Goldberg AL (1996) Importance of the ATP–ubiquitin–proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 271:26690–26697
Spencer JA, Eliazer S, Ilaria RL Jr, Richardson JA, Olson EN (2000) Regulation of microtubule dynamics and myogenic differentiation by MURF, a striated muscle RING-finger protein. J Cell Biol 150:771–784
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
Sun Z, Liu L, Liu N, Liu Y (2008) Muscular response and adaptation to diabetes mellitus. Front Biosci 13:4765–4794
Sundaram P, Pang Z, Miao M, Yu L, Wing SS (2009) USP19-deubiquitinating enzyme regulates levels of major myofibrillar proteins in L6 muscle cells. Am J Physiol Endocrinol Metab 297:E1283–E1290
Tiao G, Fagan J, Roegner V, Lieberman M, Wang JJ, Fischer JE, Hasselgren PO (1996) Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J Clin Invest 97:339–348
Tintignac LA, Lagirand J, Batonnet S, Sirri V, Leibovitch MP, Leibovitch SA (2005) Degradation of MyoD mediated by the SCF (MAFbx) ubiquitin ligase. J Biol Chem 280:2847–2856
Urso ML, Chen YW, Scrimgeour AG, Lee PC, Lee KF, Clarkson PM (2007) Alterations in mRNA expression and protein products following spinal cord injury in humans. J Physiol 579:877–892
van den Beld AW, Lamberts SW (2002) Endocrine aspects of healthy ageing in men. Novartis Found Symp 242:3–16, discussion 16–25
Vargas R, Lang CH (2008) Alcohol accelerates loss of muscle and impairs recovery of muscle mass resulting from disuse atrophy. Alcohol Clin Exp Res 32:128–137
Vary TC, Frost RA, Lang CH (2008) Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294:R1777–R1789
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA (2004) In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303:844–848
Vignaud A, Ramond F, Hourde C, Keller A, Butler-Browne G, Ferry A (2007) Diabetes provides an unfavorable environment for muscle mass and function after muscle injury in mice. Pathobiology 74:291–300
Waddell DS, Baehr LM, van den Brandt J, Johnsen SA, Reichardt HM, Furlow JD, Bodine SC (2008) The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab 295:E785–E797
Walsmith J, Roubenoff R (2002) Cachexia in rheumatoid arthritis. Int J Cardiol 85:89–99
Wang X, Hu Z, Hu J, Du J, Mitch WE (2006) Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin–proteasome pathway by defects in muscle cell signaling. Endocrinology 147:4160–4168
Welle S, Burgess K, Thornton CA, Tawil R (2009) Relation between extent of myostatin depletion and muscle growth in mature mice. Am J Physiol Endocrinol Metab 297:E935–E940
Whitman SA, Wacker MJ, Richmond SR, Godard MP (2005) Contributions of the ubiquitin–proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflugers Arch 450:437–446
Williams A, Sun X, Fischer JE, Hasselgren PO (1999) The expression of genes in the ubiquitin–proteasome proteolytic pathway is increased in skeletal muscle from patients with cancer. Surgery 126:744–749
Wray CJ, Mammen JM, Hershko DD, Hasselgren PO (2003) Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol 35:698–705
Yen HC, Elledge SJ (2008) Identification of SCF ubiquitin ligase substrates by global protein stability profiling. Science 322:923–929
Yen HC, Xu Q, Chou DM, Zhao Z, Elledge SJ (2008) Global protein stability profiling in mammalian cells. Science 322:918–923
Zeman RJ, Zhao J, Zhang Y, Zhao W, Wen X, Wu Y, Pan J, Bauman WA, Cardozo C (2009) Differential skeletal muscle gene expression after upper or lower motor neuron transection. Pflugers Arch 458:525–535
Zhao TJ, Yan YB, Liu Y, Zhou HM (2007) The generation of the oxidized form of creatine kinase is a negative regulation on muscle creatine kinase. J Biol Chem 282:12022–12029
Zhao J, Zhang Y, Zhao W, Wu Y, Pan J, Bauman WA, Cardozo C (2008) Effects of nandrolone on denervation atrophy depend upon time after nerve transection. Muscle Nerve 37:42–49
Zhao W, Pan J, Wang X, Wu Y, Bauman WA, Cardozo CP (2008) Expression of the muscle arophy factor MAFbx is suppressed by testosterone. Endocrinology 149:5449–5460
Zhao W, Qin W, Pan J, Wu Y, Bauman WA, Cardozo C (2009) Dependence of dexamethasone-induced Akt/FOXO1 signaling, upregulation of MAFbx, and protein catabolism upon the glucocorticoid receptor. Biochem Biophys Res Commun 378:668–672
Zhu E, Sassoon CS, Nelson R, Pham HT, Zhu L, Baker MJ, Caiozzo VJ (2005) Early effects of mechanical ventilation on isotonic contractile properties and MAF-box gene expression in the diaphragm. J Appl Physiol 99:747–756
Zinna EM, Yarasheski KE (2003) Exercise treatment to counteract protein wasting of chronic diseases. Curr Opin Clin Nutr Metab Care 6:87–93
Acknowledgements
We wish to thank Dr. D. Segal in the critical reading of this manuscript. APR is supported by an NH&MRC Biomedical Career Development Award (479536).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Foletta, V.C., White, L.J., Larsen, A.E. et al. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflugers Arch - Eur J Physiol 461, 325–335 (2011). https://doi.org/10.1007/s00424-010-0919-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-010-0919-9