Abstract
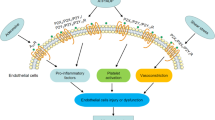
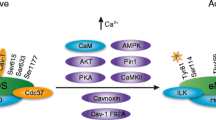
The endothelium can evoke relaxations (dilatations) of the underlying vascular smooth muscle, by releasing vasodilator substances. The best characterized endothelium-derived relaxing factor is nitric oxide (NO), which is synthesized by the endothelial isoform of nitric oxide synthase (eNOS). Endothelium-dependent relaxations involve both pertussis-toxin-sensitive Gi (e.g., responses to serotonin, sphingosine 1-phosphate, alpha2-adrenergic agonists, and thrombin) and pertussis-toxin-insensitive Gq (e.g., adenosine diphosphate and bradykinin) coupling proteins. eNOS undergoes a complex pattern of intracellular regulation, including post-translational modifications involving enzyme acylation and phosphorylation. eNOS is reversibly targeted to signal-transducing plasmalemmal caveolae where the enzyme interacts with a number of regulatory proteins, many of which are modified in cardiovascular disease states. The release of nitric oxide by the endothelial cell can be up- (e.g., by estrogens, exercise, and dietary factors) and down-regulated (e.g. oxidative stress, smoking, and oxidized low-density lipoproteins). It is reduced in the course of vascular disease (e.g., diabetes and hypertension). Arteries covered with regenerated endothelium (e.g. following angioplasty) selectively lose the pertussis-toxin-sensitive pathway for NO release which favors vasospasm, thrombosis, penetration of macrophages, cellular growth, and the inflammatory reaction leading to atherosclerosis. The unraveling of the complex interaction of the pathways regulating the presence and the activity of eNOS will enhance the understanding of the perturbations in endothelium-dependent signaling that are seen in cardiovascular disease states, and may lead to the identification of novel targets for therapeutic intervention.






Similar content being viewed by others
References
Aikawa M, Libby P (2004) The vulnerable atherosclerotic plaque pathogenesis and therapeutic approach. Cardiovasc Path 13:125–138
Balligand JL, Feron O et al (2009) eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev 89(2):481–534
Berka V, Wu G, Yeh HC et al (2004) Three different oxygen-induced radical species in endothelial nitric-oxide synthase oxygenase domain under regulation by L-arginine and tetrahydrobiopterin. J Biol Chem 279:32243–32251
Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM (2002) EDHF: bringing the concepts together. Trends Pharmacol Sci 23:374–380
Busse R, Fleming I (2003) Regulation of endothelium-derived vasoactive autacoid production by hemodynamic forces. Trends Pharmacol Sci 24:24–29
Cai H (2005) Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res 68(1):26–36
Cai H, Griendling KK et al (2003) The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24(9):471–478
Chen Z, Peng IC et al (2009) AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res 104(4):496–505
Davies PF (1995) Flow-mediated endothelial mechanotransduction. Physiol Rev 75:519–560
De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM (2000) Endothelial dysfunction in diabetes. Br J Pharmacol 130:963–974
Deanfield JE, Halcox JP et al (2007) Endothelial function and dysfunction: testing and clinical relevance. Circulation 115(10):1285–1295
Dudzinski DM, Michel T (2007) Life history of eNOS: partners and pathways. Cardiovasc Res 75(2):247–260
Dudzinski DM, Igarashi J et al (2006) The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol 46:235–276
Erwin PA, Lin AJ et al (2005) Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem 280(20):19888–19894
Erwin PA, Mitchell DA et al (2006) Subcellular targeting and differential S-nitrosylation of endothelial nitric-oxide synthase. J Biol Chem 281(1):151–157
Félétou M, Vanhoutte PM (2006) EDHF: the complete story. CRC Taylor and Francis, Boca Raton, pp 1–298
Félétou M, Vanhoutte PM (2006) Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol 291:H985–H1002
Félétou M, Vanhoutte PM (2009) EDHF: an update. Clin Sci 117(4):139–55
Feron O, Balligand JL (2006) Caveolins and the regulation of endothelial nitric oxide synthase in the heart. Cardiovasc Res 69(4):788–797
Feron O, Saldana F et al (1998) The endothelial nitric-oxide synthase-caveolin regulatory cycle. J Biol Chem 273(6):3125–3128
Fisslthaler B, Loot AE, Mohamed A, Busse R, Fleming I (2008) Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ Res 102:1520–1528
Fisslthaler B, Fleming I (2009) Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res 105(2):114–127
Fleming I, Busse R (2003) Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284:R1–R12
Forstermann U, Boissel J-P, Kleinert J (1998) Expressional control of the ‘constitutive’ isoforms of nitric oxide synthase. FASEB J 12:773–790
Fulton D, Church JE et al (2005) Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J Biol Chem 280(43):35943–35952
Fulton D, Gratton JP et al (2001) Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J Pharmacol Exp Ther 299(3):818–824
Furchgott RF, Vanhoutte PM (1989) Endothelium-derived relaxing and contracting factors. FASEB J 3:2007–2017
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376
Furuhashi M, Hotamisligil GS (2008) Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev 7:489–503
Gonzalez E, Nagiel A et al (2004) Small interfering RNA-mediated down-regulation of caveolin-1 differentially modulates signaling pathways in endothelial cells. J Biol Chem 279(39):40659–40669
Govers R, Rabelink TJ (2001) Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 280(2):F193–F206
Gratton JP, Bernatchez P et al (2004) Caveolae and caveolins in the cardiovascular system. Circ Res 94(11):1408–1417
Harrison DG (1997) Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest 100(9):2153–2157
Hess DT, Matsumoto A et al (2005) Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6(2):150–166
Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M et al (2001) Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88(2):E14–E22
Hla T, Lee MJ et al (2001) Lysophospholipids–receptor revelations. Science 294(5548):1875–1878
Icking A, Matt S et al (2005) NOSTRIN functions as a homotrimeric adaptor protein facilitating internalization of eNOS. J Cell Sci 118(Pt 21):5059–5069
Jain MK, Ridker PM (2005) Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 4(12):977–987
Katusic ZS (2001) Vascular endothelial dysfunction: does tetrahydrobiopterin play a role? Am J Physiol Heart Circ Physiol 281(3):H981–H986
Katusic ZS, d'Uscio LV et al (2009) Vascular protection by tetrahydrobiopterin: progress and therapeutic prospects. Trends Pharmacol Sci 30(1):48–54
Katusic ZS (2007) Mechanisms of endothelial dysfunction induced by aging: role of arginase I. Circ Res 101:640–641
Kojda G, Harrison D (1999) Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 43:562–571
Konig P, Dedio J et al (2005) NOSIP and its interacting protein, eNOS, in the rat trachea and lung. J Histochem Cytochem 53(2):155–164
Lee MYK, Tse HF, Siu CW, Zhu SG, Man RYK, Vanhoutte PM (2007) Genomic changes in regenerated porcine coronary arterial endothelial cells. Arterioscler Thromb Vasc Biol 27:2443–2449
Levine YC, Li GK et al (2007) Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J Biol Chem 282(28):20351–20364
Li JM, Shah AM (2004) Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol 287(5):R1014–R1030
Li PL, Gulbins E (2007) Lipid rafts and redox signaling. Antioxid Redox Signal 9(9):1411–1415
Loscalzo J, Welch G (1995) Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis 38(2):87–104
Lubos E, Handy DE et al (2008) Role of oxidative stress and nitric oxide in atherothrombosis. Front Biosci 13:5323–5344
Lüscher TF, Vanhoutte PM (1990) The endothelium: modulator of cardiovascular function. CRC, Boca Raton, pp 1–228
Martinez-Ruiz A, Villanueva L et al (2005) S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci USA 102:8525–8530
Miller VM, Duckles SP (2008) Vascular actions of estrogens: functional implications. Pharmacol Rev 60:210–241
Miller VM, Vanhoutte PM (1988) Enhanced release of endothelium-derived factors by chronic increases in blood flow. Am J Physiol 255:H446–H451
Miller VM, Vanhoutte PM (1991) Progesterone and modulation of endothelium-dependent responses in canine coronary arteries. Am J Physiol 261:R1022–R1027
Moens AL, Kass DA (2006) Tetrahydrobiopterin and cardiovascular disease. Arterioscler Thromb Vasc Biol 26(11):2439–2444
Moncada S (1997) Nitric oxide in the vasculature: physiology and pathophysiology. Ann N Y Acad Sci 811:60–67
Radi R (2004) Nitric oxide, oxidations, and protein tyrosine nitration. Proc Natl Acad Sci USA 101:4003–4008
Rubanyi GM, Vanhoutte PM (1986) Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor(s). Am J Physiol 250:H822–H827
Rubanyi GM, Lorenz RR, Vanhoutte PM (1985) Bioassay of endothelium-derived relaxing factor(s). Inactivation by catecholamines. Am J Physiol 249:H95–H101
Rubanyi GM, Romero JC, Vanhoutte PM (1986) Flow-induced release of endothelium-derived relaxing factor. Am J Physiol 250:H1145–H1149
Schilling K, Opitz N et al (2006) Translocation of endothelial nitric-oxide synthase involves a ternary complex with caveolin-1 and NOSTRIN. Mol Biol Cell 17(9):3870–3880
Schroder E, Eaton P (2008) Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: issues and considerations. Curr Opin Pharmacol 8(2):153–159
Sessa WC (2004) eNOS at a glance. J Cell Sci 117(Pt 12):2427–2429
Shaul PW (2002) Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol 64:749–774
Shaul PW (2003) Endothelial nitric oxide synthase, caveolae and the development of atherosclerosis. J Physiol 547(Pt 1):21–33
Skidgel RA (2002) Proliferation of regulatory mechanisms for eNOS: an emerging role for the cytoskeleton. Am J Physiol Lung Cell Mol Physiol 282(6):L1179–L1182
Stamler JS, Lamas S, Fang FC (2001) Nitrosylation: the prototypic redox-based signaling mechanism. Cell 106:675–683
Steuhr DJ (1997) Structure-function aspects in the nitric oxide synthases. Ann Rev Pharmacol Toxicol 37:339–359
Stocker R, Keaney JF Jr (2004) Role of oxidative modifications in atherosclerosis. Physiol Rev 84:1381–1478
Su Y, Edwards-Bennett S et al (2003) Regulation of endothelial nitric oxide synthase by the actin cytoskeleton. Am J Physiol Cell Physiol 284(6):C1542–C1549
Thomas SR, Witting PK et al (2008) Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 10(10):1713–1765
Vanhoutte PM (2008) Arginine and arginase: eNOS double crossed? Circ Res 102:866–868
Vanhoutte PM, Tang EHC (2008) Endothelium-dependent contractions: when a good guy turns bad. J Physiol 586:5295–5303
Vanhoutte PM (2009) How we learned to say NO. Arterioscl Thromb Vasc Biol 29:1156–1160
Vanhoutte PM, Félétou M, Taddei S (2005) Endothelium-dependent contractions in hypertension. Br J Pharmacol 144:449–458
Vanhoutte PM, Shimokawa H, Tang EHC, Félétou M (2009) Endothelial dysfunction and vascular disease. Acta Physiol 196:193–222
Wolin MS (2009) Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol 296(3):H539–H549
Acknowledgment
The authors thank Mr. Robert R. Lorenz for the expert help in preparing the figures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michel, T., Vanhoutte, P.M. Cellular signaling and NO production. Pflugers Arch - Eur J Physiol 459, 807–816 (2010). https://doi.org/10.1007/s00424-009-0765-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-009-0765-9