Abstract
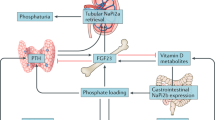
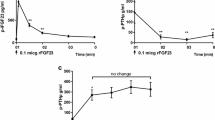
The phosphaturic activity of intact, full-length, fibroblast growth factor-23 (FGF-23) is well documented. FGF-23 circulates as the intact protein and as fragments generated as the result of proteolysis of the full-length protein. To assess whether short fragments of FGF-23 are phosphaturic, we compared the effect of acute, equimolar infusions of full-length FGF-23 and various FGF-23 fragments carboxyl-terminal to amino acid 176. In rats, intravenous infusions of full-length FGF-23 and FGF-23 176–251 significantly and equivalently increased fractional phosphate excretion (FE Pi) from 14 ± 3 to 32 ± 5% and 15 ± 2 to 33 ± 2% (p < 0.001), respectively. Chronic administration of FGF-23 176–251 reduced serum Pi and serum concentrations of 1α,25-dihydroxyvitamin D. Shorter forms of FGF-23 (FGF-23 180–251 and FGF-23 184–251) retained phosphaturic activity. Further shortening of the FGF-23 carboxyl-terminal domain, however, abolished phosphaturic activity, as infusion of FGF-23 206–251 did not increase urinary phosphate excretion. Infusion of a short fragment of the FGF-23 molecule, FGF-23 180–205, significantly increased FE Pi in rats and reduced serum Pi in hyperphosphatemic Fgf-23 −/− knockout mice. The activity of FGF-23 180–251 was confirmed in opossum kidney cells in which the peptide reduced Na+-dependent Pi uptake and enhanced internalization of the Na+-Pi IIa co-transporter. We conclude that carboxyl terminal fragments of FGF-23 are phosphaturic and that a short, 26-amino acid fragment of FGF-23 retains significant phosphaturic activity.




Similar content being viewed by others
References
ADHR Consortium (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348
Bai X, Miao D, Li J, Goltzman D, Karaplis AC (2004) Transgenic mice overexpressing human fibroblast growth factor 23 (R176Q) delineate a putative role for parathyroid hormone in renal phosphate wasting disorders. Endocrinology 145:5269–5279
Bai XY, Miao D, Goltzman D, Karaplis AC (2003) The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem 278:9843–9849
Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B (2005) An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet 14:385–390
Berndt TJ, Bielesz B, Craig TA, Tebben PJ, Bacic D, Wagner CA, O’Brien S, Schiavi S, Biber J, Murer H, Kumar R (2006) Secreted frizzled-related protein-4 reduces sodium-phosphate co-transporter abundance and activity in proximal tubule cells. Pflugers Arch 451:579–587
Berndt TJ, Schiavi S, Kumar R (2005) “Phosphatonins” and the regulation of phosphorus homeostasis. Am J Physiol Renal Physiol 289:F1170–F1182
Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine MA, Kumar R, Schiavi SC (2001) FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun 284:977–981
Braun S, auf dem Keller U, Steiling H, Werner S (2004) Fibroblast growth factors in epithelial repair and cytoprotection. Philos Trans R Soc Lond B Biol Sci 359:753–757
Campos M, Couture C, Hirata IY, Juliano MA, Loisel TP, Crine P, Juliano L, Boileau G, Carmona AK (2003) Human recombinant endopeptidase PHEX has a strict S1’ specificity for acidic residues and cleaves peptides derived from fibroblast growth factor-23 and matrix extracellular phosphoglycoprotein. Biochem J 373:271–279
Cancilla B, Davies A, Cauchi JA, Risbridger GP, Bertram JF (2001) Fibroblast growth factor receptors and their ligands in the adult rat kidney. Kidney Int 60:147–155
Cancilla B, Ford-Perriss MD, Bertram JF (1999) Expression and localization of fibroblast growth factors and fibroblast growth factor receptors in the developing rat kidney. Kidney Int 56:2025–2039
Chen P, Toribara T, Warnner H (1956) Microdetermination of phosphorus. Anal Chem 28:1756–1758
Eswarakumar VP, Lax I, Schlessinger J (2005) Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 16:139–149
Führ J, Kazmarczyk J, Krüttgen CD (1955) Eine einfache colorimetrische Methode zur Inulin-Bestimmung für Nieren-clearance-untersuchungen bei StoffwechselGesunden und Diabetikern. Klin Wochenschr 33:729–730
Fukumoto S, Yamashita T (2002) Fibroblast growth factor-23 is the phosphaturic factor in tumor-induced osteomalacia and may be phosphatonin. Curr Opin Nephrol Hypertens 11:385–389
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M (2006) Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123
Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB (2004) Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology 145:3087–3094
Poduslo JF, Curran GL, Peterson JA, McCormick DJ, Fauq AH, Khan MA, Wengenack TM (2004) Design and chemical synthesis of a magnetic resonance contrast agent with enhanced in vitro binding, high blood–brain barrier permeability, and in vivo targeting to Alzheimer’s disease amyloid plaques. Biochemistry 43:6064–6075
Razzaque MS, Lanske B (2006) Hypervitaminosis D and premature aging: lessons learned from Fgf23 and Klotho mutant mice. Trends Mol Med 12:298–305
Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B (2006) Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J 20:720–722
Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P (2003) FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 112:683–692
Schiavi SC, Kumar R (2004) The phosphatonin pathway: new insights in phosphate homeostasis. Kidney Int 65:1–14
Schiavi SC, Moe OW (2002) Phosphatonins: a new class of phosphate-regulating proteins. Curr Opin Nephrol Hypertens 11:423–430
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T (2004) FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T (2004) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113:561–568
Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T (2001) Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98:6500–6505
Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T (2002) Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 143:3179–3182
Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T (2004) FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun 314:409–414
Sitara D, Razzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Juppner H, Lanske B (2004) Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol 23:421–432
Sitara D, Razzaque MS, St-Arnaud R, Taguchi T, Erben RG, Lanske B (2006) Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23 null animals. Am J Pathol 169:2161–2170
Steiling H, Werner S (2003) Fibroblast growth factors: key players in epithelial morphogenesis, repair and cytoprotection. Curr Opin Biotechnol 14:533–537
Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E (2004) Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 36:579–581
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774
White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ (2001) Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int 60:2079–2086
White KE, Jonsson KB, Carn G, Hampson G, Spector TD, Mannstadt M, Lorenz-Depiereux B, Miyauchi A, Yang IM, Ljunggren O, Meitinger T, Strom TM, Juppner H, Econs MJ (2001) The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab 86:497–500
Yamashita T, Konishi M, Miyake A, Inui K, Itoh N (2002) Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem 277:28265–28270
Yamashita T, Yoshioka M, Itoh N (2000) Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun 277:494–498
Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S (2002) Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87:4957–4960
Yan X, Yokote H, Jing X, Yao L, Sawada T, Zhang Y, Liang S, Sakaguchi K (2005) Fibroblast growth factor 23 reduces expression of type IIa Na+/Pi co-transporter by signaling through a receptor functionally distinct from the known FGFRs in opossum kidney cells. Genes Cells 10:489–502
Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, Linhardt RJ, Ornitz DM, Mohammadi M, White KE (2005) Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology 146:44647–4656
Acknowledgment
Supported by NIH grant DK-65830, a grant from Genzyme, and the Harvard School of Dental Medicine
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berndt, T.J., Craig, T.A., McCormick, D.J. et al. Biological activity of FGF-23 fragments. Pflugers Arch - Eur J Physiol 454, 615–623 (2007). https://doi.org/10.1007/s00424-007-0231-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-007-0231-5