Abstract
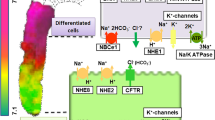
DRA (down regulated in adenoma, SLC26A3) is an anion exchanger that mediates electroneutral NaCl absorption in the ileum and proximal colon together with NHE3 (Na/H exchanger isoform 3), and that is involved in duodenal and possibly pancreatic bicarbonate secretion. Thus, its chloride and bicarbonate affinities are important for both processes. [Cl]i and pHi transients were measured using MQAE and BCECF. HEK293 cells stably expressing DRA were exposed to 0 mM Cl at various [HCO3] (9 to 51 mM, at 5% CO2 or 15 to 57 mM, at pH 7.5) to determine the HCO3 affinity. After intracellular Cl depletion, 10, 30, and 90 mM Cl were readded at various [HCO3]s to determine the relative Cl and HCO3 affinities. The k 0.5 for extracellular HCO3 is between 18.5 and 32.8 mM. Cl and HCO3 compete with similar affinities for transport by DRA. DRA activity is independent of pHo between 7.0 and 7.75. DRA is activated by alkaline pHi. Competition of Cl and HCO3 does not significantly impair NaCl absorption, because in the ileum and colon, luminal Cl is comparably high. Activation at alkaline pHi supports functional coupling of DRA and NHE3 by the subapical pHi. In the distal pancreatic ductal system, luminal HCO3 is high compared to luminal Cl. Under these conditions, competition of Cl and HCO3 is difficult to reconcile with a role of DRA in Cl reabsorption in exchange for HCO3. Our data, thus, provide indirect evidence against a role of DRA in pancreatic HCO3 secretion.






Similar content being viewed by others
Notes
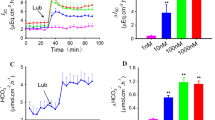
Upon switching to 0 mM Cl, there was some delay (∼15 s) in the effect on the MQAE signal compared to the BCECF signal; this difference did not occur when Cl was readded. The phenomenon is not understood at present.
In this case, the k 0.5 has no unit because it describes the ratio of Cl to HCO3.
The kinetic analysis of Figs. 5 and 6 was initially done using the Hill equation. In each case, the Hill coefficient was close to 1 and fitting the data to the Hill equation was statistically not superior than fitting them to Michaelis–Menten kinetics. Therefore, Michaelis–Menten kinetics are presented.
References
Alvarez BV, Kieller DM, Quon AL, Markovich D, Casey JR (2004) Slc26a6: a cardiac chloride-hydroxyl exchanger and predominant chloride-bicarbonate exchanger of the mouse heart. J Physiol 561:721–734
Boyarsky G, Ganz MB, Sterzel RB, Boron WF (1988) pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of \({\text{\rm HCO}}^{ - }_{3}\). Am J Physiol 255:C844–C856
Chernova MN, Jiang L, Shmukler BE, Schweinfest CW, Blanco P, Freedman SD, Stewart AK, Alper SL (2003) Acute regulation of the SLC26A3 congenital chloride diarrhoea anion exchanger (DRA) expressed in Xenopus oocytes. J Physiol 549:3–19
Hoglund P, Haila S, Socha J, Tomaszewski L, Saarialho-Kere U, Karjalainen-Lindsberg ML, Airola K, Holmberg C, de la Chapelle A, Kere (1996) Mutations of the Down-regulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet 14:316–319
Knickelbein R, Aronson PS, Atherton W, Dobbins JW (1983) Sodium and chloride transport across rabbit ileal brush border. I. Evidence for Na–H exchange. Am J Physiol 245:G504–G510
Knickelbein R, Aronson PS, Schron CM, Seifter J, Dobbins JW (1985) Sodium and chloride transport across rabbit ileal brush border. II. Evidence for Cl–HCO3 exchange and mechanism of coupling. Am J Physiol 249:G236–G245
Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S (2004) Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol 6:343–350
Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S (2002) A molecular mechanism for aberrant CFTR-dependent \({\text{\rm HCO}}^{ - }_{3}\) transport in cystic fibrosis. EMBO J 21:5662–5672
Koncz C, Daugirdas JT (1994) Use of MQAE for measurement of intracellular [Cl−] in cultured aortic smooth muscle cells. Am J Physiol 267:H2114–H2123
Lamprecht G, Baisch S, Schoenleber E, Gregor M (2005) Transport properties of the human intestinal anion exchanger DRA (down regulated in adenoma) in transfected HEK293 cells. Pflugers Arch 449:479–490
Lamprecht G, Heil A, Baisch S, Lin-Wu E, Yun CC, Kalbacher H, Gregor M, Seidler U (2002) The down regulated in adenoma (dra) gene product binds to the second PDZ domain of the NHE3 kinase A regulatory protein (E3KARP), potentially linking intestinal \({{\text{\rm CI}}^{{\text{ - }}} } \mathord{\left/ {\vphantom {{{\text{CI}}^{{\text{ - }}} } {{\text{HCO}}^{ - }_{3} }}} \right. \kern-\nulldelimiterspace} {{\text{\rm HCO}}^{ - }_{3} }\) exchange to Na+/H+ exchange. Biochemistry 41:12336–12342
Melvin JE, Park K, Richardson L, Schultheis PJ, Shull G (1999) Mouse Down-regulated in Adenoma (DRA) is an intestinal Cl/HCO3 exchanger and is up-regulated in colon of mice lacking the NHE3 Na+/H+ exchanger. J Biol Chem 274:22855–22861
Mugharbil A, Knickelbein RG, Aronson PS, Dobbins JW (1990) Rabbit ileal brush-border membrane Cl-HCO3 exchanger is activated by an internal pH-sensitive modifier site. Am J Physiol 259:G666–G670
Nader M, Lamprecht G, Classen M, Seidler U (1994) Different regulation by pHi and osmolarity of the rabbit ileum brush-border and parietal cell basolateral anion exchanger. J Physiol 481:605–615
O’Reilly C, Winpenny J, Argent B, Gray M (2000) Cystic fibrosis transmembrane conductance regulator currents in guinea pig pancreatic duct cells: inhibition by bicarbonate ions. Gastroenterology 118:1187–1196
Poulsen JH, Fischer H, Illek B, Machen TE (1994) Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A 91:5340–5344
Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE (1998) Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19:282–285
Steward MC, Ishiguro H, Case RM (2005) Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol 67:377–409
Turnberg LA (1973) Absorption and secretion of salt and water by the small intestine. Digestion 9:357–381
Turnberg LA, Bieberdorf FA, Morawski SG, Fordtran JS (1970) Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Invest 49:557–567
Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M (2005) Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol 288:C957–C965
Yun CHC, Lamprecht G, Foster DV, Sidor A (1998) NHE3 Kinase A regulatory protein (E3KARP) binds the epithelial brush border Na/H exchanger, NHE3, and the cytoskeletal protein ezrin. J Biol Chem 273:25856–25863
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the Link to the electronic supplementary material.
Figa
Chloride transients measured at constant pHo (7.5). HEK/EGFP-DRA cells were exposed to 0 mM extracellular Cl and various HCO3 concentrations (15, 27 and 57 mM HCO3 at 2.5%, 5% and 10 %CO2 respectively). At 900 seconds chloride was readded at various concentrations while keeping the HCO3 concentration constant (Panel A 10 mM Cl, panel B 30 mM Cl, panel C 90 mM Cl)
Figb
pHi transients measured at constant pHo (7.5). HEK/EGFP-DRA cells were exposed to 0 mM extracellular Cl and various HCO3 concentrations (15, 27 and 57 mM HCO3 at 2.5%, 5% and 10 %CO2 respectively). At 600 seconds chloride was readded at various concentrations while keeping the HCO3 concentration constant (Panel A 10 mM Cl, panel B 30 mM Cl, panel C 90 mM Cl)
Rights and permissions
About this article
Cite this article
Lamprecht, G., Schaefer, J., Dietz, K. et al. Chloride and bicarbonate have similar affinities to the intestinal anion exchanger DRA (down regulated in adenoma). Pflugers Arch - Eur J Physiol 452, 307–315 (2006). https://doi.org/10.1007/s00424-006-0049-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0049-6