Abstract
Introduction
Hernia repair with prosthetic meshes represents one of the most common surgical procedures in the field of surgery. This intervention is always associated with an ensuing inflammatory response, angiogenesis and fibrotic encapsulation forming a foreign body granuloma (FBG) around the mesh fibres. Several studies have described this inflammatory reaction by characterising inflammatory cell infiltrate around the FBG after mesh explantation. However, very little is known about the real-time progression of such an inflammatory response. The aim of this study was to investigate the feasibility of monitoring the ongoing inflammatory response to mesh implantation using bioluminescence in vivo.
Materials and methods
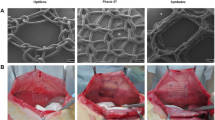
Three luciferase transgenic mice strains (FVB/N-Tg(Vegfr2-luc)-Xen, BALB/C-Tg(NFκB-RE-luc)-Xen and Tg(INS/EpRE-Luc)T20Rbl) were used. Mice were anaesthetized with 2 % isoflurane, and two incisions were made on the left and right sides of the abdomen of the mice. A 1-cm2 propylene mesh was implanted subcutaneously in the right incision wound of each mouse, and the left wound served as control. Two hundred microliters of D-luciferin was injected into the mice, and bioluminescence measurements were done prior to the surgical intervention and subsequently every 3 days. After mesh explantation, histological analysis was done. Statistical analysis was done using prism GraphPad software.
Results
Bioluminescence results revealed different time points of maximum signal for the different mice strains. VEGFR2 gene expression peaked on day 6, NFkB on day 12 and ARE on day 3 post mesh implantation. We also observed much higher bioluminescent signal around the FBG surrounding the mesh as compared to the control wound, with p < 0.05 for all the different mice strains.
Conclusion
Our results prove the possibility of monitoring the inflammatory reaction after mesh implantation in vivo using bioluminescence signal release. This provides a novel method of accessing and accurately describing the ongoing inflammatory response over a given period of time.




Similar content being viewed by others
References
Conze J, Binneboesel M, Junge K, Schumpelick V (2010) Incisional hernia—how do I do it?—standard surgical approach. Chirurg 81:192–200
Junge K, Binneboesel M, Rosch R, Otto J, Kaemmer D, Schumpelick V, Klinge U (2009) Impact of proinflammatory cytokine knockout on mesh integration. J Investig Surg 22:256–262
Klosterhalfen B, Klinge U, Schumpelick V (1998) Functional and morphological evaluation of different polypropylene-mesh modifications for abdominal wall repair. Biomaterials 19:2235–2246
Junge K, Klinge U, Rosch R, Klosterhalfen B, Schumpelick V (2002) Functional and morphologic properties of a modified mesh for inguinal hernia repair. World J Surg 26:1472–1480
Leber GE, Garb JL, Alexander AI, Reed WP (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133:378–382
Anderson JM, Rodriguez A, Chang DT (2008) Foreign body reaction to biomaterials. Semin Immunol 20:86–100
Junge K, Binneboesel M, von Trotha KT, Rosch R, Klinge U, Neumann UP, Jansen PL (2012) Mesh biocompatibility: effects of cellular inflammation and tissue remodelling. Langenbecks Arch Surg 397:255–270
Charo IF, Ransohoff RM (2006) Mechanisms of disease—the many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354:610–621
Esche C, Stellato C, Beck LA (2005) Chemokines: key players in innate and adaptive immunity. J Investig Dermatol 125:615–628
Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N (2003) Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol 73:713–721
Wruck CJ, Streetz K, Pavic G, Goetz ME, Tohidnezhad M, Brandenburg L-O, Varoga D, Eickelberg O, Herdegen T, Trautwein C et al (2011) Nrf2 Induces interleukin-6 (IL-6) expression via an antioxidant response element within the IL-6 promoter. J Biol Chem 286:4493–4499
Dendorfer U, Oettgen P, Libermann TA (1994) Multiple regulatory Elements in the interleukin-6 Gene mediate Induction by prostaglandins, cyclic-amp, and lipopolysaccharide. Mol Cell Biol 14:4443–4454
Schmidt T, Carmeliet P (2010) BLOOD-VESSEL FORMATION Bridges that guide and unite. Nature 465:697–699
Zhang N, Fang ZX, Contag PR, Purchio AF, West DB (2004) Tracking angiogenesis induced by skin wounding and contact hypersensitivity using a Vegfr2-luciferase transgenic mouse. Blood 103:617–626
Lauer G, Sollberg S, Cole M, Flamme I, Sturzebecher J, Mann K, Krieg T, Eming SA (2000) Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Investig Dermatol 115:12–18
Mori R, Kondo T, Ohshima T, Ishida Y, Mukaida N (2002) Accelerated wound healing in tumor necrosis factor receptor p55-deficient mice with reduced leukocyte infiltration. Faseb J 16:963–974
Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R, Chrzanowska-Wodnicka M (2011) Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin alpha(v)beta(3). Blood 118:2015–2026
Contag PR, Olomu IN, Stevenson DK, Contag CH (1998) Bioluminescent indicators in living mammals. Nat Med 4:245–247
Wang YL, Iyer M, Annala A, Wu L, Carey M, Gambhir SS (2006) Noninvasive indirect imaging of vascular endothelial growth factor gene expression using bioluminescence imaging in living transgenic mice. Physiol Genomics 24:173–180
Carlsen H, Moskaug JO, Fromm SH, Blomhoff R (2002) In vivo imaging of NF-kappa B activity. J Immunol 168:1441–1446
Jaiswal AK (2004) Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 36:1199–1207
Ronicke V, Risau W, Breier G (1996) Characterization of the endothelium-specific murine vascular endothelial growth factor receptor-2 (Flk-1) promoter. Circ Res 79:277–285
Wruck CJ, Fragoulis A, Gurzynski A, Brandenburg L-O, Kan YW, Chan K, Hassenpflug J, Freitag-Wolf S, Varoga D, Lippross S, Pufe T (2011) Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann Rheum Dis 70:844–850
Dohlen G, Odland HH, Carlsen H, Blomhoff R, Thaulow E, Saugstad OD (2008) Antioxidant activity in the newborn brain: a luciferase mouse model. Neonatology 93:125–131
Schumpelick V, Conze J, Klinge U (1996) Preperitoneal mesh repair of incisional hernias—a comparative retrospective study. Chirurg 67:1028–1035
Bellón JM, Bujan J, Contreras L, Hernando A, Jurado F (1994) Macrophage response to experimental implantation of polypropylene prostheses. Eur Surg Res 26(1):46–53
Junge K, Klinge U, Prescher A, Giboni P, Niewiera M, Schumpelick V (2001) Elasticity of the anterior abdominal wall and impact for reparation of incisional hernias using mesh implants. Hernia J Hernias Abdom Wall Surg 5:113–118
Welty G, Klinge U, Klosterhalfen B, Kasperk R, Schumpelick V (2001) Functional impairment and complaints following incisional hernia repair with different polypropylene meshes. Hernia J Hernias Abdom Wall Surg 5:142–147
Klosterhalfen B, Klinge U, Henze U, Bhardwaj R, Conze J, Schumpelick V (1997) Morphologic correlation of functional abdominal wall mechanics after mesh implantation. Langenbecks Arch Surg 382(2):87–94, German
Weyhe D, Schmitz I, Belyaev O, Grabs R, Müller KM, Uhl W, Zumtobel V (2006) Experimental comparison of monofile light and heavy polypropylene meshes: less weight does not mean less biological response. World J Surg 30(8):1586–1591
Chang C-T, Ho T-Y, Lin H, Liang J-A, Huang H-C, Li C-C, Lo H-Y, Wu S-L, Huang Y-F, Hsiang C-Y (2012) 5-Fluorouracil induced intestinal mucositis via nuclear factor-kappa B activation by transcriptomic analysis and in vivo bioluminescence imaging. Plos One 7
Southern MM, Brown PE, Hall A (2006) Luciferases as reporter genes. Methods Mol Biol 323:293–305
Gray KD, Simovic MO, Blackwell TS, Christman JW, May AK, Parman KS, Chapman WC, Stain SC (2006) Activation of nuclear factor kappa B and severe hepatic necrosis may mediate systemic inflammation in choline-deficient/ethionine-supplemented diet-induced pancreatitis. Pancreas 33:260–267
Baeuerle PA, Baltimore D (1988) Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-kappa-b transcription factor. Cell 53:211–217
Mi FL, Tan YC, Liang HF, Sung HW (2002) In vivo biocompatibility and degradability of a novel injectable-chitosan-based implant. Biomaterials 23:181–191
Liang HC, Chang Y, Hsu CK, Lee MH, Sung HW (2004) Effects of crosslinking degree of an acellular biological tissue on its tissue regeneration pattern. Biomaterials 25:3541–3552
Vijayasekaran S, Fitton JH, Hicks CR, Chirila TV, Crawford GJ, Constable IJ (1998) Cell viability and inflammatory response in hydrogel sponges implanted in the rabbit cornea. Biomaterials 19:2255–2267
La Flamme KE, Popat KC, Leoni L, Markiewicz E, La Tempa TJ, Roman BB, Grimes CA, Desai TA (2007) Biocompatibility of nanoporous alumina membranes for immunoisolation. Biomaterials 28:2638–2645
Sato A, Klaunberg B, Tolwani R (2004) In vivo bioluminescence imaging. Comp Med 54:631–634
Ho T-Y, Chen Y-S, Hsiang C-Y (2007) Noninvasive nuclear factor-kappa B bioluminescence imaging for the assessment of host-biomaterial interaction in transgenic mice. Biomaterials 28:4370–4377
Contag CH, Contag PR, Mullins JI, Spilman SD, Stevenson DK, Benaron DA (1995) Photonic detection of bacterial pathogens in living hosts. Mol Microbiol 18:593–603
Junge K, Klinge U, Klosterhalfen B, Mertens PR, Rosch R, Schachtrupp A, Ulmer F, Schumpelick V (2002) Influence of mesh materials on collagen deposition in a rat model. J Investig Surg 15:319–328
Klinge U, Klosterhalfen B, Conze J, Limberg W, Obolenski B, Ottinger AP, Schumpelick V (1998) Modified mesh for hernia repair that is adapted to the physiology of the abdominal wall. Eur J Surg 164:951–960
Acknowledgements
The project is co-funded by the European Union (European Regional Development Fund—investing in your future) and the German federal state North Rhine-Westphalia (NRW).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fet, N., Alizai, P.H., Fragoulis, A. et al. In vivo characterisation of the inflammatory reaction following mesh implantation in transgenic mice models. Langenbecks Arch Surg 399, 579–588 (2014). https://doi.org/10.1007/s00423-014-1192-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-014-1192-8