Abstract
Background
We had previously described a left lateral segment hyper-reduction technique capable of sizing the graft according to the volume of the abdominal cavity of the recipient.
Aim
The purpose of our study was to evaluate our 14-year live-donor liver transplantation experience with in situ graft hyper-reduction in children under 10 kg of weight.
Patients and methods
Between January 1997 and May 2011, we performed 881 liver transplants. Two hundred and seventy-seven (n = 277) involved pediatric recipients, of which 102 (37 %) were from live donors. Thirty-five (n = 35) patients under 10 kg of weight underwent hyper-reduced living donor liver transplants. There were 21 females (60 %) and 14 males (40 %), with a median age of 12 months (range 3–23) and a median weight of 7.7 kg (range 5.6–10).
Results
Median operative time was 350 min (range 180–510). Median cold ischemia time was 180 min (range 60–300). Twenty-six (n = 26) patients required intraoperative transfusion of blood products. There were 49 postoperative complications involving 26 patients (74 % morbidity rate). One-, 3-, and 5-year survival rates were 87, 79, and 74 %, respectively. Twenty-eight patients are currently alive.
Conclusions
Hyper-reduced grafts provide an alternative approach for low-weight pediatric recipients. The relatively high immediate postoperative morbidity could be related to the complexity of these patients.
Similar content being viewed by others
Introduction
Pediatric liver transplantation in the very young patients remains one of the most complex surgical procedures. The technical difficulties associated with the almost unavoidable donor–recipient size discrepancy have prompted multiple approaches to overcome the challenges of graft reduction, complex vascular reconstructions, and intricate abdominal closures [1–3]. Several techniques such as reduced graft, split, and living donor transplantation have been developed over the years. Unfortunately, even the greatest reductions remain unable to fashion grafts suitable for recipients with very low body weights.
We had previously described a technique capable of minimizing left lateral segment grafts while maintaining their biliary–vascular pedicles intact [2]. Such hyper-reduction allowed for the tailoring of grafts according to the recipient abdominal volume (especially the antero-posterior diameter) and the donor liver size. In the current series, we outline our 14-year live-donor liver transplantation experience in children under 10 kg of weight with such ultrasound-guided in situ graft hyper-reduction. We propose this hyper-reduction technique as an effective alternative to address the complex problem of liver transplantation in children under 10 kg of weight.
Patients and methods
Study population
Between January 1997 and May 2011, 881 consecutive liver transplants were performed at the Hospital Italiano de Buenos Aires. Two hundred and seventy-seven involved pediatric recipients, of which 102 (37 %) were from live donors. Forty-four (79 %) patients weight less than 10 kg. Thirty-five of these patients underwent hyper-reduced living donor liver transplants, six were transplanted using reduced cadaveric livers, and three used pediatric cadaveric donors.
According to the Argentinean law, living donors should be genetically related until second line blood (grandparents, father, mother, brother, or uncles) and foreign patients (23 of 35 patients less than 10 kg in weight in this series) can only be transplanted using living-related donors because they cannot be listed for cadaveric transplants. Donors were the mother in 26 cases, the father in 6, and an uncle in 3.
The hyper-reduced living donor recipients were 21 females (60 %) and 14 males (40 %), with a median age of 12 months (range 3–23) and a median weight of 7.7 kg (range 5.6–10). The underlying cause of liver failure was biliary atresia in 28 cases, Alagille syndrome in 3, familial cholestasis in 2, urea cycle disorder in 1, and autoimmune hepatitis in 1. All transplants were performed electively. Thirty-one patients were admitted to the hospital at the time of transplantation. Four had been admitted earlier because of clinical manifestations of their underlying disease. Immunosuppression in the first five cases was based on cyclosporine and steroids. The remaining 30 received tacrolimus and steroids. The last 15 patients received induction with thymoglobulin followed by low doses of tacrolimus. Postoperative complications were classified according to the Dindo system [4]. Dindo grades III–V were considered to be major complications. Data were collected from electronic medical records as well as from the liver transplantation database.
Donor evaluation
During donor evaluation, when the volume of the left lateral segment was greater than 5 % of the recipient's total body weight, a hyper-reduction “a la carte” was performed. Because these children are very lightweight and according to the literature [5, 6], we use grafts of up to 5 % of recipient's body weight in order to expand the pool of potential donors. This technique has the advantage that both surgeries are performed simultaneously, giving us the possibility to reduce the graft a la carte according to the space availability in the recipient's cavity.
Donor evaluation included a general assessment of the patient condition and study of liver volume by CT scan. The vascular anatomy is assessed by splanchnic angiography (hepatic artery and portal vein anatomy) and dynamic enhanced CT scan (hepatic vein anatomy). The anatomy of the recipient is evaluated by dynamic enhanced CT scan with vascular reconstruction.
Procurement technique
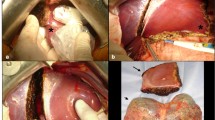
A J-shaped or midline supraumbilical incision was performed based on individual donor anatomical findings. The liver was subsequently mobilized by taking down the falciform, left triangular, and left coronary ligaments. Intraoperative ultrasound was used to identify vascular and biliary structures. The left hepatic artery was dissected along its course from the common hepatic artery. Left portal vein was dissected and segment 1 branches were ligated. Left bile duct was dissected and cut at the right side of the umbilicus fistula. The ligament of Arantius was subsequently transected, and the confluence of the middle and left hepatic veins dissected. The left hepatic vein was purposefully not mobilized and kept as short as possible in order to avoid post-implantation torsions. The liver parenchyma was transected 1 cm to the right of the falciform ligament, isolating segments 2 and 3 from segment 4. Clips, ties, and biological glues were used in the process. Segments 2 and 3 were then hyper-reduced, tailoring the volume and shape of the graft to the specific intraoperative dimensions of the recipient abdominal cavity (reduction on demand a la carte). The section line of the peripheral hepatic parenchyma is performed in situ under ultrasound guidance prior to procurement of the graft allowed us to preserve the biliary–vascular pedicle intact (Fig. 1) and to identify areas of ischemia prior to procurement. Transecting along vertical and horizontal planes (Fig. 2) provided a cubical graft shaped like a “chocolate brownie” (Fig. 3). The left portal vein, left hepatic artery, and left hepatic vein were clamped and transected. The specimen was perfused with preservation fluid in the back table and prepared for implantation.
Implantation technique
The recipient surgery entailed a total hepatectomy with preservation of the inferior vena cava (IVC). The graft's left hepatic vein was implanted onto the recipient IVC. The graft requires a rotation to be implanted. This rotation placed the main section line lying on the anterior face of the IVC. The site of implantation in the IVC had to be prepared with great care. The technical aspects of its preparation are described in Fig. 4. The anastomosis was constructed with running 6-0 polypropylene sutures (Fig. 5).
A vertical incision is made along the IVC starting at the lower end of the ostium of the right hepatic vein. Subsequently, an oblique incision is made on the anterior surface of the IVC connecting the previous incision to a point close to the right side of the ostium of the middle hepatic vein. The third incision connected the remaining two sites. The resulting opening is two times larger than the ostium of the donor left hepatic vein
In order to avoid postoperative mass effect compression of the vascular structures, we performed a complete lymphadenectomy of the porta hepatis. The portal vein anastomosis was fashioned with running 7-0 polypropylene sutures, as close as possible to the confluence of the recipient's splenic and mesenteric veins. The arterial reconstruction was performed in all instances with 10-0 polypropylene sutures under microscopic magnification. The bile duct was anastomosed onto a Roux-en-Y jejunal limb with 7-0 or 8-0 polypropylene or PDS sutures using the same technique that left living donor liver transplant. We did not use any biliary stents or drains. Occasionally, we performed the bilioenteric anastomosis prior to arterial anastomosis of the allograft as a precautionary measure to prevent thrombosis due to temporary rotation of the graft. Doppler ultrasound before and after closing the abdominal wall is routinely performed in order to check permeability of vascular anastomoses. The criteria to assess the patency of vascular structures using Doppler ultrasound were as follows: hepatic artery—peak systolic velocity >30 cm/s and end diastolic velocity >5 cm/s; portal vein—flow velocity >5 cm/s, hepatopetal flow with respiratory wave oscillations and absence of echogenic material inside the vein; hepatic vein—visualization of the vein with hepatofugal non-filiform flow, respiratory wave oscillations, and absence of echogenic material inside the vein. In case of diagnostic doubt, a digital splanchnic angiography is performed. During postoperatory course, daily laboratory monitoring of liver function (liver function tests, PT, factor V, and lactic acid) and Doppler ultrasound was performed.
Statistical analysis
Categorical variables were expressed as percentages. Continuous variables were reported as median and range (r). Survival was calculated using the Kaplan–Meier method. Statistical analysis was performed with SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Median recipient operative time was 350 min (range 180–510), with cold ischemia time of 180 min (range 60–300). Median donor operative time was 180 min (range 150–270). The donor portal vein had to be lengthened with donor's veins graft (three inferior mesenteric veins and one saphenous vein) in four recipients.
There were three intraoperative portal vein thromboses. Two were successfully treated by performing thrombectomy with a Fogarty catheter. The third case required intraoperative placement of an expandable stent. Twenty-six recipients required intraoperative transfusion of blood products. Twenty-five received packed red blood cells (median 220 ml, range 40–400). Twenty-two required fresh frozen plasma (median 300 ml, range 100–900). Despite hyper-reduction and due to the small recipient cavity size, a prosthetic (Goretex) mesh was needed for abdominal closure in six recipients. All patients required postoperative-assisted mechanical ventilation for a median of 5 days (range 1–22). Median length of stay in the intensive care unit was 20 days (range 4–69), with a median total length of hospital stay of 33 days (range4–72). There were 49 postoperative complications involving 26 patients (74 % morbidity rate) as follows: 19 grade II, 7 grade IIIa, 18 grade IIIb, 2 grade IV, and 3 grade V.
Two patients developed liver failure: one associated with impaired venous outflow and the other because of portal and arterial thrombosis. Both were retransplanted using pediatrics diseased donors (graft reduction was performed in both cases).
There were two postoperative deaths: one because of subarachnoid hemorrhage at postoperative day (POD) 4 and the other after retransplantation at POD 24. Median follow-up was 37 months (range 4–210). One-, 3-, and 5-year survival rates were 87, 79, and 74 %, respectively.
There were five late deaths. Four patients died of EBV-related lymph-proliferative disorders 7, 9, 34, and 49 months after transplantation. The fifth patient died of sepsis on postoperative month 24.
Twenty-eight patients are currently alive. Eight developed late complications. Five developed biliary strictures at 1, 2, 3.5, 4, and 7 years. Four of them were successfully treated with percutaneous dilatation and one is being treated with antibiotics alone. Two patients required removal of the Goretex mesh at 9 months and 3 years after transplantation because of chronic infection. One developed a portal vein thrombosis with esophageal varices that were successfully treated with banding. One patient developed mesenteric vein thrombosis requiring extensive small bowel resection. After a period of 3 months with total parenteral nutrition, he has full recovery.
Concerning donors, none of them required transfusion of blood products. Only one patient developed postoperative complications (biloma that required percutaneous drainage) and there were no mortality.
Discussion
Multiple and many times unavoidable technical difficulties characterize transplantation in the very young patients. Recipients under 1 year of age constitute 29 % of all pediatric liver recipients, with the number increasing to 38 % by 2 years [3]. In our current series, we describe our 14-year experience with graft hyper-reduction as a response to the lack of size-compatible grafts [2, 7]. Although the liver constitutes 2 % of the total body weight of adults, at birth it is as high as 5 % [8]. When the ratio of graft size to recipient weight is greater than 5 %, graft reduction may be necessary to prevent a large-for-size syndrome (LFS). LFS is characterized by inadequate tissue oxygenation and graft compression [5, 9, 10] and is associated with increased vascular and biliary complications as well as accelerated acute cellular rejection [11].
The lack of suitable donors for recipients with low body weights prompted the development of various techniques aimed at expanding the potential organ pool [12–14]. In 1984, Bismuth and Housin [15] described liver size reduction, a technique (as originally described) limited to instances of donor–recipient body weight disparities of fourfold. Strong et al. [16], in 1988, allowed for greater weight disparities with the use of adult left lateral segments for pediatric recipients. Split- and live-donor liver transplantation, variations using segments 2 and 3, continued to be of limited use in cases of children weighing less than 10 kg [5, 6]. In the 1990s, Housin et al. [17] described a new size reduction technique using only segment 2. This reduction (resecting segment 3), performed after the implantation of the left lateral segment, was characterized by technical difficulties associated with hemostatic disorders of the revascularized graft. Strong et al. [7] further described a case of a deceased donor left lateral segment graft in which segment 2 was resected in the back table (guided by a guide wire in the lumen of the left hepatic vein) and segment 3 subsequently implanted. Mentha et al. [17] reported the implantation of segment 2 from a cadaveric liver after the resection of segment 3. In this case, the removal of segment 3 was guided by injecting methylene blue into the segment 3 branch of the portal vein. Srinivasan et al. later described a series of six cases of segment 3 grafts from deceased donors in patients with acute liver failure, stating that “this technique also could be potentially extended to LDLT” [18].
Based on this initial work and on the so-called hepatectomy a la carte described by Bismuth et al. [15], in 1997, we started to develop a technique of further reducing what Bismuth and others defined as reduced grafts. Our so-called hyper-reduction technique aimed at adapting the volume of the graft to the size of the recipient abdominal cavity by performing an “in situ” tailoring of the graft prior to procurement from the donor [2]. The use of intraoperative ultrasound was essential in our approach, since the vascular elements could be easily identified “in vivo,” allowing for a safe transection plane. Our procedure provided non-monosegmental grafts inclusive of segments 2 and 3. Potential areas of ischemia were readily identified at the time of procurement, something impossible to do when performing the reduction in a back table. The cubical shape was a result of our transection planes avoiding vasculobiliary structures. The anteroposterior diameter of the recipient abdominal cavity (least affected by ascites and native liver enlargement) was our major guide for graft size. We did not rely on preoperative volumetry since we observed a 30–50 % increase in graft volume associated with post-implantation congestion. Our technique furthermore allowed for the acceptance of donors that would have been considered too big to split (results not published in the current series).
The procurement of monosegmental liver grafts has several drawbacks. It is technically demanding since the plane of dissection has to be continuously reevaluated during the resection. It is also usually associated with long vascular pedicles, prone to vascular complications such as torsions, kinks, and twists. The technique described in our series involves only two planes of transection, and the resulting liver graft encompasses parts of the both segments 2 and 3. As thoroughly demonstrated in our current series, and contrary to previous reports [4, 10, 16–19], there is no need to carry out anatomically precise monosegmental resections. Since we did not perform any type of hilar dissection of segments 2–3, our approach avoided biliary complications associated with unknown or unusual anatomical configurations. Ultrasound guidance allowed for the intraoperative identification of vascular–biliary pedicles. Our major challenge was not associated with the small volume of the grafts, but with the meticulous technique required to perform the delicate vascular and biliary reconstructions. After an early trial period, we chose to routinely anastomose the donor left hepatic vein to the recipient inferior vena cava, avoiding the serious complication of venous outflow impairment. Table 1 summarizes previous hyper-reduction reports in pediatric liver transplantation, mostly involving monosegmental grafts. Of ten indexed publications on the topic, only three groups apply this technique with living-related donor; the rest used cadaveric donors. Our series, in addition to being the largest, is also the only one addressing hyper-reduction a la carte. Table 2 summarized the advantages and disadvantages of monosegmental and hyper-reduced grafts.
Previous studies had shown that early transplantation provides for improved catch-up growth [20]. When considering the fact that until recently low-weight pediatric patients were rarely transplanted because of both their size and the lack of size-matched donors, leading to a mortality rate close to 100 %, we believe that the reported morbidity and mortality are not unexpected in this challenging population. Our long-term survival rates are comparable to those of other series, even among those who did not require hyper-reduction [21–26].
The hyper-reduction technique had a twofold beneficial effect on the pediatric waiting list. It increased the number of potential recipients by allowing smaller children to be transplanted and it augmented the number of potential donors by including individuals that were previously rejected because of their size. Furthermore, it also provided for an increased number of organs in countries such as ours where laws allow only close relatives to qualify as live donors. The relatively high immediate postoperative morbidity could be related to the complexity of these patients.
References
Grabhorn E, Schulz A, Helmke K et al (2004) Short- and long-term results of liver transplantation in infants aged less than 6 months. Transplantation 78:235–241
de Santibanes E, McCormack L, Mattera J et al (2000) Partial left lateral segment transplant from a living donor. Liver Transpl 6:108–112
Noujaim HM, Mayer DA, Buckles JA et al (2002) Techniques for and outcome of liver transplantation in neonates and infants weighing up to 5 kilograms. J Pediatr Surg 37:159–164
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Kiuchi T, Kasahara M, Uryuhara K et al (1999) Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation 67:321–327
Tanaka A, Tanaka K, Tokuka A et al (1996) Graft size-matching in living related partial liver transplantation in relation to tissue oxygenation and metabolic capacity. Transpl Int 9:15–22
Strong R, Lynch S, Yamanaka J et al (1995) Monosegmental liver transplantation. Surgery 118:904–906
Kliegman R, Nelson WE (2007) Nelson textbook of pediatrics, 18th edn. Saunders, Philadelphia
Kasahara M, Kaihara S, Oike F et al (2003) Living-donor liver transplantation with monosegments. Transplantation 76:694–696
Kasahara M, Uryuhara K, Kaihara S et al (2003) Monosegmental living donor liver transplantation. Transplant Proc 35:1425–1426
Tanaka K, Uemoto S, Tokunaga Y et al (1993) Surgical techniques and innovations in living related liver transplantation. Ann Surg 217:82–91
Raia S, Nery JR, Mies S (1989) Liver transplantation from live donors. Lancet 2:497
Strong RW, Lynch SV, Ong TH et al (1990) Successful liver transplantation from a living donor to her son. N Engl J Med 322:1505–1507
Broelsch CE, Whitington PF, Emond JC et al (1991) Liver transplantation in children from living related donors. Surgical techniques and results. Ann Surg 214:428–437, discussion 37–9
Bismuth H, Houssin D (1984) Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery 95:367–370
Strong R, Ong TH, Pillay P et al (1988) A new method of segmental orthotopic liver transplantation in children. Surgery 104:104–107
Houssin D, Soubrane O, Boillot O (1992) Orthotopic liver transplantation with a reduced-size graft: an ideal compromise in pediatrics? Surgery 111:532–542
Mentha G, Belli D, Berner M et al (1996) Monosegmental liver transplantation from an adult to an infant. Transplantation 62:1176–1178
Srinivasan P, Vilca-Melendez H, Muiesan P et al (1999) Liver transplantation with monosegments. Surgery 126:10–12
Enne M, Pacheco-Moreira LF, Cerqueira A et al (2004) Liver transplantation with monosegment from a living donor. Pediatr Transplant 8:189–191
Alonso G, Duca P, Pasqualini T et al (2004) Evaluation of catch-up growth after liver transplantation in children with biliary atresia. Pediatr Transplant 8:255–259
Diamond IR, Fecteau A, Millis JM et al (2007) Impact of graft type on outcome in pediatric liver transplantation: a report from Studies of Pediatric Liver Transplantation (SPLIT). Ann Surg 246:301–310
Attia MS, Stringer MD, McClean P et al (2008) The reduced left lateral segment in pediatric liver transplantation: an alternative to the monosegmental graft. Pediatr Transplantation 12:696–700
Soubrane O, Houssin D, Pitre J, Dousset B, Bernard O, Chapuis Y (1994) Extrafascial hyper-reduction of the hepatic graft. J Am Coll Surg 178(2):139–143
Oh SH, Kim KM, Lee YJ et al (2010) Long-term outcomes of pediatric living donor liver transplantation at a single institution. Pediatr Transplantation 14:870–878
Neto JS, Carone E, Pugliese V et al (2007) Living donor liver transplantation for children in Brazil weighing less than 10 kilograms. Liver Transpl 13:1153–1158
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ardiles, V., Ciardullo, M.A., D’Agostino, D. et al. Transplantation with hyper-reduced liver grafts in children under 10 kg of weight. Langenbecks Arch Surg 398, 79–85 (2013). https://doi.org/10.1007/s00423-012-1020-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-012-1020-y