Abstract
Purpose
Carbohydrate ingestion prior and during exercise attenuates exercise-induced interleukin-6. This investigation examined if an analogous effect was evident for interleukin-6 and hepcidin response when carbohydrates were ingested post-exercise.
Methods
In a crossover design, 11 well-trained endurance athletes completed two experimental trials. Participants completed an 8 × 3 min interval running session at 85 % vVO2peak followed by 5 h of monitored recovery. During this period, participants were provided with two 1.2 g kg−1 carbohydrate beverages at either an early feeding time (immediately post-exercise and 2 h post-exercise) or delayed feeding time (2 h post-exercise and 4 h post-exercise). Venous blood samples were collected pre-, immediately post-, 3 and 5 h post-exercise. Samples were analysed for Interleukin-6, serum iron, serum ferritin and hepcidin.
Results
Interleukin-6 was significantly elevated (p = 0.004) immediately post-exercise compared to baseline for both trials. Hepcidin levels were significantly elevated at 3 h post-exercise (p = 0.001) and 5 h post-exercise (p = 0.002) compared to baseline levels in both trials, with no significant difference between the two conditions and any time point. Serum iron was significantly increased from baseline to immediately post-exercise (p = 0.001) for both trials, with levels decreasing by 3 h (p = 0.025) and 5 h post-exercise (p = 0.001). Serum ferritin levels increased immediately post-exercise compared to baseline (p = 0.006) in both conditions.
Conclusions
The timing and ingestion of post-exercise carbohydrate ingestion do not appear to impact post-exercise interleukin-6 and hepcidin responses; this is likely a result of the interval running task inducing an inflammatory response and subsequent up-regulation of hepcidin.
Similar content being viewed by others
Introduction
The replenishment of muscle glycogen stores post-exercise is optimally achieved through carbohydrate (CHO) intake, with the timing of consumption suggested to be 1.0–1.2 g CHO kg body mass−1 (Burke 2010) within the first 2 h after exercise cessation (Ivy et al. 1988). However, alternative research has suggested that exercise undertaken with low muscle glycogen content enhances intracellular endurance training responses (for review see Yeo et al. 2008; Burke et al. 2011), and has been the basis of the recently described nutritional training strategy known as, ‘Train Low, Compete High’ (TLCH). Employing such a strategy encourages athletes to train with low muscle glycogen content, but to compete with high muscle glycogen stores to promote optimal exercise performance (Yeo et al. 2008; Burke et al. 2011). With this in mind, a potential strategy to reduce muscle glycogen stores is to withhold CHO intake during the first hours of recovery post-exercise, in an attempt to reduce the total glycogen storage between sessions that are less than 8 h apart (Burke 2010).
Training with low muscle glycogen stores has been reported to result in post-exercise elevations to the inflammatory cytokine interleukin-6 (IL-6), suggesting that IL-6 may have a role in hepatic glucose homeostasis (Keller et al. 2001; Steensberg et al. 2001). Thus, in an attempt to reduce IL-6 expression post-exercise, research has investigated the role of CHO ingestion during exercise (Nieman et al. 1998). Previously, Nieman et al. (1998) had endurance runners complete a 2.5-h run whilst ingesting 250 ml of a 6 % CHO solution or a sweetened (sugarless) placebo beverage every 15 min. The results indicated that ingestion of CHO attenuated IL-6 levels post-exercise by approximately 40 %. In addition, Robson-Ansley et al. (2011) also reported lower post-exercise IL-6 levels with a smaller total amount of CHO, after an 8 % CHO solution was ingested at 2 ml kg−1 every 20 min during a 2-h run at 60 % vVO2max, which preceded a 5-km running time trial. Therefore, it appears that the rate of IL-6 release from contracting skeletal muscle is at least partly dependent on CHO availability. However, it should be noted that the ingestion of CHO post-exercise, and any subsequent impact on exercise-induced increases in IL-6, is yet to be investigated.
In addition to its role in glucose homeostasis, IL-6 is an up-regulator of the hepatic hormone hepcidin, which is a key regulator of iron metabolism (Nemeth et al. 2004a) through its action on the cellular iron exporter, ferroportin (Fpn) (Lymboussaki et al. 2003; Nemeth et al. 2004b). Elevations in hepcidin have been reported to prevent the recycling of iron from macrophages and the absorption of iron at the level of the gut (Nemeth et al. 2004b). Research has established that peak elevations in hepcidin expression occur approximately 3 h post-exercise, subsequent to the well-established elevations in IL-6 immediately post-exercise (Peeling et al. 2009a). With this in mind, it is suggested that there may exist a period of reduced dietary iron absorption and recycling during the post-exercise period. Furthermore, given the established link between CHO and IL-6, it is possible that if an athlete had low CHO availability post-exercise, as a result of delaying their post-exercise CHO intake, this may exacerbate the effect on post-exercise IL-6 and the subsequent hepcidin response. Regardless, to date there has been minimal consideration for the aforementioned association between inflammation, hepcidin and delayed CHO intake post-exercise. Consequently, the aim of this study was to examine the impact of withholding immediate post-exercise CHO ingestion on the subsequent inflammatory and hepcidin responses, subsequent to the completion of endurance exercise.
Methods
Participants
Eleven well-trained male endurance runners and triathletes participated in this study [mean ± SD age: 31 ± 6 years; height: 1.8 ± 0.6 m; body mass: 72.1 ± 10.2 kg; peak oxygen uptake (VO2peak): 63.0 ± 4.4 ml kg−1 min−1]. Determination of the sample size was attained via a power analysis using customised computer software (GPOWER Version 3.1.5, Department of Psychology, Bonn University, Bonn Germany), with data from previous investigations that measured similar variables (Badenhorst et al. 2014). The power analysis suggested a sample size of 10, for an expected power of 0.89 with an alpha level of p ≤ 0.05. The experimental protocols were approved by the University of Western Australia’s Institutional Review Board, conforming to the Declaration of Helsinki on the use of human subjects. All participants were informed of the study protocols, requirements, benefits and risks and informed consent was obtained prior to the commencement of the investigation. Volunteers who presented with food allergies (e.g. lactose intolerance, nuts, celiac disease) or compromised iron stores (serum ferritin <30 µg L−1: see Peeling et al. 2014) were excluded from participation.
Experimental overview
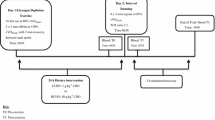
All participants completed three separate testing sessions at the exercise physiology laboratory of the School of Sport Science, Exercise and Health, at the University of Western Australia. The preliminary testing session served as a familiarisation trial for the participants to become accustomed to the laboratory environment and the equipment to be used during the subsequent experimental trials. The preliminary session concluded with a running-based graded exercise test (GXT) to exhaustion, used to determine each individual’s peak oxygen uptake (VO2peak). The velocities corresponding to 60 and 85 % of VO2peak were then used to set the warm-up and interval running speed, used in the two subsequent experimental interval running sessions.
In the 24 h prior to each experimental trial, participants consumed a standardised diet [8 g CHO kg−1 of body mass (BM)], containing 64 % CHO, 13 % protein and 17 % fat, for a total energy content of 14,710 (±1832) kJ. The diet provided was fashioned to the individual’s BM, with pre-packaged food and drinks being supplied to the participants to minimise the amount of preparation required. Dietary compliance was assessed by the participants returning all emptied containers and food delivery bags on the morning of each interval running session.
A repeated-measures, counterbalanced, crossover design was used, with each experimental session separated by a minimum of 7 days, and all sessions were conducted at the same time of day to minimise the influence of circadian variation (06:30 am ± 1 h). The sessions commenced with measurement of the participant’s body mass (measured on August Sauter GmbH Scale., West Germany) and collection of a pre-exercise blood sample, before they completed a 5-min warm-up at 60 % vVO2peak, followed by 5 min of structured dynamic stretching. Following this, the interval running session commenced, consisting of 8 × 3 min repeats at 85 % vVO2peak with a 90-s active recovery at 60 % vVO2peak between repetitions (2:1 work-to-rest ratio). A motorised treadmill (VR3000, NuryTech Inc., Korea) was used. This interval running task has previously been used in research investigating post-exercise hepcidin responses (Sim et al. 2014; Badenhorst et al. 2014). During the exercise trials, heart rate (HR) and ratings of perceived exertion (RPE) were monitored at the completion of each 3-min workload. A capillary blood sample for lactate (BLa) analysis was collected after the fourth and eighth interval repetition. After completing the interval running task, a 5-min cool-down run at 60 % vVO2peak was completed, then participants rested in the laboratory for the next 5 h. Water was provided during the running sessions to minimise any exercise haemoconcentration effects. The volume consumed during the initial running trial was measured post-exercise and then replicated in the subsequent trial. During the 5h recovery period, participants received either an early CHO (ECHO) or delayed CHO (DCHO) feeding in the form of a 1.2 g CHO kg−1 beverage (12 ml kg−1, 10 % CHO beverage) containing a mixture of Gatorade (Gatorade©, Schweppes Australia) and Polyjoule (Polyjoule©, Nutricia Australia) powders. The CHO beverages were constructed relative to the individuals’ BM on the day of each trial. For the ECHO trial, participants received their CHO beverages at 15 and 120 min post-exercise: for the DCHO trial, at 120 and 240 min post-exercise. Participants ingested the CHO beverage over a 30-min period. To control total fluid intake with the provision of a CHO nutritional beverage post-exercise, water intake was restricted for the 30 min before and after each CHO feeding (Areta et al. 2013). The water volume ingested during the recovery periods of the first session was also recorded and replicated during the subsequent trial to maintain consistency. Urine loss during the 5-h recovery period was measured via changes in body mass before and after emptying the bladder, after accounting for fluid ingestion (Convertino et al. 1996). In addition, venous blood samples were collected throughout each trial: on arrival (baseline), at the conclusion of the interval running session (post-exercise), after 3 h of post-exercise recovery (3 h post-exercise), and finally after 5 h of post-exercise recovery (5 h post-exercise).
Experimental procedures
Graded exercise test (GXT)
The GXT was conducted on a motorised treadmill (VR3000, NuryTech Inc., Korea) using 3-min exercise and 1-min rest periods. The initial speed was set to 12 km h−1, with subsequent increments of 1 km h−1 until voluntary exhaustion. The treadmill was set to a 1 % gradient to simulate common outdoor conditions (Jones and Doust 1996). Expired air was analysed for O2 and CO2 concentrations using Ametek Gas Analysers (Applied Electrochemistry, SOV S-3A/1 and COV CD-3A, Pittsburgh, PA). The gas analysers were calibrated pre-test and verified post-test with certified gravimetric gas mixtures (BOC Gases, Chatswood, Australia). During the test, ventilation was recorded at 15-s intervals via a turbine ventilometer (Vacu-Med Ventura, California), also calibrated prior to the test and verified after exercise using a 1-L syringe in accordance with the manufacturer’s specifications. The VO2peak was obtained by summing the four highest consecutive 15-s VO2 values obtained throughout the test.
Blood sampling and analysis
During the GXT and high-intensity interval exercise sessions, capillary blood samples were collected for BLa analysis using a Lactate Pro analyser (ARKRAY, Japan). Venous blood samples were collected from a forearm antecubital vein with the participant first lying down for 10 min to control for postural shifts in plasma volume. These samples were collected using a 22-gauge needle into two 8.5-mL SST Gel separator tubes and one 4-ml EDTA collection tube (BD vacutainer, Australia). Immediately after collection, the fresh plasma samples from the EDTA tubes were taken to the Royal Perth Hospital pathology laboratory for haemoglobin (Hb) concentration and haematocrit (Hct) analysis. These data were used for the determination of percentage changes in plasma volume as a result of the exercise task (Dill and Costill 1974). The SST samples were allowed to clot for 60 min at room temperature before subsequently being centrifuged at 10 °C and 3000 rpm (1.4 rcf) for 10 min. The serum supernatant was divided into 1-mL aliquots and stored at −80 °C until further analysis. Once all the blood samples were collected, the frozen serum was transported to Royal Perth Hospital pathology laboratory for analysis of circulating levels of IL-6, serum iron and serum ferritin. The remaining frozen serum samples were sent to Radboud University Medical Centre (Nijmegen, The Netherlands) for serum hepcidin-25 analysis.
Serum IL-6 was analysed using a commercially available ELISA (Quantikine HS, R&D Systems, Minneapolis, USA) with an assay range of 0.38–10 ng L−1. The coefficient of variation (CV) for IL-6 determination at 0.49 and 2.78 ng L−1 was 9.6 and 7.2 %, respectively. Serum iron levels were determined using an iron reagent (Sentinel Diagnostics, Milano, Italy) and an Architect analyser (c1600210). The CV for iron determination at 10.50 and 42.96 µmol L1 was 3.15 and 1.00 %, respectively. Serum ferritin levels were determined using an Architect analyser (1SR06055) and a ferritin reagent (Abbott Diagnostics, Illinois, USA). The CV for ferritin determination at 5.48, 34.46, 187.52 and 403.87 µg L−1 was 5.05, 4.36, 4.44 and 3.91 %, respectively. Serum hepcidin-25 measurements were performed (www.hepcidinanalysis.com, Nijmegen, The Netherlands) by a combination of weak cation exchange chromatography and time-of-flight mass spectrometry (WCX-TOF MS) (Kroot et al. 2010). An internal standard (synthetic hepcidin-24; custom made Peptide International Inc.) was used for quantification (Laarakkers et al. 2013). Peptide spectra were generated on a Microflex LT matrix-enhanced laser desorption/ionisation TOF MS platform (Bruker Daltonics). Plasma hepcidin-25 concentrations were expressed as nmol L−1 (nM). The median reference level of serum hepcidin-25 (Dutch population) is 4.5 nM for men; 2.0 nM for premenopausal women; and 4.9 nM for postmenopausal women (www.hepcidinanalysis.com, accessed on February 7th, 2015).
Perceived exertion and heart rate
Throughout the experimental trials, RPE was recorded using the Borg perceptual scale (Borg 1982) encompassing the anchor points 6 (very, very light) through to 20 (maximal exertion). Heart rate was measured continuously via a Garmin HR monitor (Garmin 210, USA).
Statistical analysis
Results are presented as mean and standard deviation (±SD) unless otherwise stated. Results were analysed in the IBM Statistical Package for Social Sciences (IBM SPSS version 19.0). A repeated-measures ANOVA was used to analyse the time, condition and interaction (condition*trial) effects of ECHO vs. DCHO on the inflammatory, hepcidin and iron-related responses post-exercise. Post-hoc, least significant differences (LSD) paired samples t tests were used to determine where specific trial differences existed. The alpha level used was p ≤ 0.05. Where appropriate, Cohen’s d effect sizes were calculated to suggest data trends and were interpreted as d < 0.39 (small); d = 0.40–0.69 (moderate); d > 0.7 (large) (Hopkins 2005). Only moderate and large effect sizes are presented here.
Results
Physiological responses
Overall mean values of HR, RPE and BLa data for the interval running trials are presented in Table 1. No significant differences were reported for HR (p = 0.536), BLa (p = 0.486) and RPE (p = 0.307) between the ECHO and DCHO trials. Percentage changes in plasma volume recorded immediately post-exercise (p = 0.949) and urine loss across the 5-h recovery period (p = 0.384) were also not different between the ECHO and DCHO conditions.
Interleukin-6
No significant condition (p = 0.344) or interaction (p = 0.431) effects were recorded for IL-6 (Table 2). However, a significant time effect (p = 0.004) was recorded for IL-6 in both the ECHO and DCHO trials. Within both trials, there was a significant increase in IL-6 from baseline to immediately post-exercise (ECHO: p = 0.033, DCHO: p = 0.003). No significant difference was recorded for the relative change in IL-6 (baseline to immediately post-exercise) between the ECHO and DCHO trials (p = 0.432).
Hepcidin
Significant time (p = 0.001), but not condition (p = 0.069) or interaction (p = 0.077) effects were recorded for serum hepcidin between the ECHO and DCHO trials (Table 2). In ECHO and DCHO, there was a significant increase in serum hepcidin from baseline to 3 h post-exercise (ECHO: p = 0.004; DCHO: p = 0.001), and from baseline to 5 h post-exercise (ECHO: p = 0.002; DCHO: p = 0.003). There was no difference in serum hepcidin levels between 3 and 5 h post-exercise for both ECHO (p = 0.278) and DCHO (p = 0.345).
Iron parameters
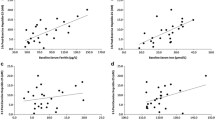
There were significant time (p = 0.001) and interaction (p = 0.001) effects for serum iron between the ECHO and DCHO trials (Table 2). The ECHO and DCHO serum iron levels significantly increased from baseline to immediately post-exercise (both p = 0.001), before significantly decreasing from immediately post-exercise to 3 h (ECHO: p = 0.001, DCHO: p = 0.025) and 5 h (both p = 0.001). In both ECHO and DCHO trials, serum iron levels at 5 h post-exercise were significantly lower than baseline (ECHO: p = 0.001, DCHO: p = 0.047) and 3 h post-exercise (both p = 0.001). In addition, in the ECHO trial, the 3-h post-exercise serum iron levels were significantly lower than baseline values (p = 0.002). There were no significant condition (p = 0.317) or interaction (p = 0.278) effects for serum ferritin between the ECHO and DCHO trials (Table 2). However, a significant time (p = 0.006) effect indicated that in the ECHO and DCHO trials, serum ferritin levels were significantly elevated immediately post-exercise, as compared to baseline (ECHO: p = 0.042, DCHO: p = 0.009) (Table 2).
Discussion
The results from this investigation demonstrate that following a 24-h standardised diet (8 g CHO kg−1 BM) and a standardised interval running session (8 × 3 min at 85 % vVO2peak), significant increases in IL-6 and serum hepcidin were evident post-exercise for both DCHO and ECHO trials and occurred irrespective of the CHO (1.2 g CHO kg−1 BM) ingestion timing in the post-exercise recovery period.
During exercise, IL-6 is reported to increase exponentially, with peak levels attained immediately post-exercise (Nieman et al. 1998; Robson-Ansley et al. 2007; Peeling et al. 2009b), as found here in both trials. No significant differences in IL-6 levels were evident between the ECHO and DCHO trials either pre- or immediate post-exercise. Previously, variables such as exercise mode, duration, intensity and the 24-h pre-exercise diet have been reported to influence the magnitude of IL-6 response following exercise (Steensberg et al. 2001; Helge et al. 2003; Fischer 2006); here, these factors were matched for each trial, so that the post-exercise timing of CHO ingestion could be investigated without these potentially confounding influences.
The IL-6 response following exercise has been demonstrated to up-regulate the iron regulatory hormone, hepcidin (Kemna et al. 2005). Peak post-exercise hepcidin levels are usually attained approximately 3 h following exercise, subsequent to significant elevations in IL-6 (Peeling et al. 2009a; Sim et al. 2012; Badenhorst et al. 2014). Elevations in hepcidin levels in the post-exercise recovery period have been suggested as a potential mechanism for altered iron metabolism within athletes (Roecker et al. 2005; Peeling et al. 2008; Latunde-Dada 2013), due to the hormone’s influence on the Fpn cellular iron export channels located on the basolateral intestinal enterocytes and macrophage cell surface (Nemeth et al. 2004b). Consequently, an individual may be susceptible to a reduction in iron recycling and iron absorption during the post-exercise period (Peeling et al. 2009a). In the current investigation, there were no significant differences in serum hepcidin expression post-exercise between trials, regardless of the timing of CHO ingestion during recovery. As such, it is likely that after completing the interval running task, the similar elevations seen in IL-6 in both the DCHO and ECHO trials resulted in an analogous exercise-induced stimulus for the signalling of hepcidin expression, resulting in significantly increased levels of hepcidin after both trials.
Acute bouts of exercise are known to cause marked physiological, metabolic and hormonal responses, with elevations in IL-6 post-exercise suggested to mediate some of these effects (Fischer 2006). With regard to metabolic effects, IL-6 has been suggested to act in a counter-regulatory hormone-like manner, up-regulating glucose production in the liver, to help sustain energy output for physical activity (Steensberg et al. 2000). Having compromised muscle glycogen stores as a result of prolonged or intense exercise are reported to invoke greater IL-6 levels immediately post-exercise, whereas normal muscle glycogen status, or the ingestion of CHO prior and during exercise, attenuates this IL-6 response (Nieman et al. 1998; Steensberg et al. 2001; Nieman et al. 2003; Febbraio et al. 2003). Previously, Ivy et al. (1988) have demonstrated that following prolonged exercise; muscle glycogen synthesis rates are 45 % slower when CHO ingestion is delayed by 2 h post-exercise, as compared to immediate CHO ingestion. This is suggested to occur due to reductions in post-exercise glucose transport in the absence of CHO (Cartee et al. 1989; Goodyear et al. 1990).Therefore, the immediate consumption of CHO (1.2 g CHO kg−1) at the conclusion of exercise is thought to be highly beneficial in aiding recovery from strenuous exercise via rapid replenishment of muscle glycogen stores (Burke et al. 2011). However, regardless of this recommendation, the results here demonstrated that both the immediate and delayed ingestion of CHO was ineffective in attenuating the IL-6 and hepcidin response following high-intensity interval running exercise. This suggests that the post-exercise ingestion of CHO may be an ineffective protocol for regulating iron metabolism.
Despite this finding, it remains possible that the ingestion and timing of CHO after an initial exercise session may still be beneficial if the individuals muscle glycogen stores are significantly reduced following exercise. Ronsen et al. (2002) showed that a recovery time of 3 h between two bouts of strenuous endurance exercise resulted in significantly greater levels of IL-6 when compared to an identical training day with 6-h recovery between exercise bouts. These authors concluded that the augmentation of IL-6 with the shorter recovery period was likely the result of glycogen depletion in the working muscle, with increases in IL-6 serving as a signal of energy shortage (Ronsen et al. 2002). The aforementioned research suggests that significant depletion of muscle glycogen stores as a result of intense exercise protocols, reduced recovery time between exercise sessions or the withholding CHO intake, may be the primary factor that will influence the magnitude of the exercise induce IL-6 response. Within the current study, the lack of significant difference in the IL-6 and subsequent hepcidin responses when post-exercise feeding was re-scheduled (early to delayed) could be a result of insufficient muscle glycogen depletion from the exercise protocol that was utilised. In addition, the starting muscle glycogen stores (whilst not directly assessed) were likely to be elevated due to the standardised diet (8 g CHO kg−1 BM) on a minimal activity day 24 h prior to the commencement of the experimental trials. Therefore, it is likely that the combination of a standardised diet recommended for athletes (Burke et al. 2011) and an exercise protocol that had previously been used in research investigating the hepcidin response post-exercise (Sim et al. 2014; Badenhorst et al. 2014) may not have provided enough stress to the participants muscle glycogen stores. Hence, the timing of the post-exercise feeding had less significance on the post-exercise IL-6 and iron metabolism responses.
In support of previous literature (Fallon 2001; Peeling et al. 2009a, c), serum ferritin levels were significantly elevated immediately post-exercise in both DCHO and ECHO trials. These elevations are expected due to exercise inducing an inflammatory reaction in the reticuloendothelial system (RE) with increased ferritin synthesis and cell membrane damage in ferritin storage tissues resulting in the subsequent release of ferritin into plasma (Pattini et al. 1990). In addition, the elevations in serum ferritin may result from exercise-induced haemolysis (Weight et al. 1991) leading to the leakage of tissue ferritin into the circulation. Significant increases in serum iron were also reported post-exercise in both DCHO and ECHO trials, suggesting an exercise-induced haemolytic reaction, characterised by increases in free haemoglobin (Hb), serum haptoglobin (Hp) and serum iron (Buchman et al. 1998; Peeling et al. 2009b), occurred in response to the interval running. After exercise increases in serum iron, that is bound to the circulating protein transferrin, may have a supplementary effect on the hepcidin response, as a result of the homeostatic regulation of iron, whereby increases in serum transferrin bound iron levels result in the up-regulation of hepcidin (Kroot et al. 2011). As mentioned previously, elevated hepcidin levels can affect the recycling of iron from the macrophages and the absorption of dietary iron, and as such, there may exist an interaction between the degree of post-exercise haemolysis incurred and the ability to absorb dietary iron following exercise. However, the increase in serum hepcidin via increases in serum iron (homeostatic) as a result of a haemolytic reaction following exercise and the impact on iron metabolism remains to be investigated. Regardless exercise-induced inflammatory up-regulation of hepcidin is considered to be the primary contributor to a negative outcome on an athlete’s iron metabolism.
Limitations
It should be acknowledged that muscle glycogen content was not quantified during this investigation. In addition, during this investigation there was no control group (i.e. a group where no CHO was provided) to compare to the ECHO and DCHO trials conducted within this investigation. The addition of such a control group may have been beneficial to assess if the ingestion of CHO post-exercise did influence inflammatory and hepcidin responses post-exercise, regardless of timing.
Conclusion
In summary, following an intense interval running session, the ingestion of the recommended amount of CHO (1.2 g CHO kg BM) immediately post-exercise, or delayed by 2 h post-exercise, did not have a significant effect on the post-exercise IL-6 or hepcidin responses. This may have been the result of the interval running task inducing an IL-6 response prior to the ingestion of CHO at the completion of exercise. As a result of the exercise-induced IL-6, serum hepcidin was up-regulated 3 h post-exercise, and was not influenced by the timing of CHO ingestion in the post-exercise period.
Abbreviations
- ANOVA:
-
Analysis of variance
- BLa:
-
Blood lactate
- BM:
-
Body mass
- CHO:
-
Carbohydrate
- CO2 :
-
Carbon dioxide
- CV:
-
Coefficient of variance
- DCHO:
-
Delayed carbohydrate feeding trial
- ECHO:
-
Early carbohydrate feeding trial
- Fpn:
-
Ferroportin
- GXT:
-
Graded exercise test
- Hb:
-
Haemoglobin
- Hct:
-
Haematocrit
- HR:
-
Heart rate
- Hp:
-
Haptoglobin
- IL-6:
-
Interleukin-6
- O2 :
-
Oxygen
- RE:
-
Reticuloendothelial system
- RPE:
-
Rating of perceived exertion
- SD:
-
Standard deviation
- TLCH:
-
Train low, compete high
- VO2peak :
-
Peak oxygen uptake
- vVO2peak :
-
Velocity at peak oxygen uptake
References
Areta JL, Burke LM, Ross ML et al (2013) Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol 591:2319–2331. doi:10.1113/jphysiol.2012.244897
Badenhorst CE, Dawson B, Goodman C et al (2014) Influence of post-exercise hypoxic exposure on hepcidin response in athletes. Eur J Appl Physiol 114:951–959. doi:10.1007/s00421-014-2829-6
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Buchman AL, Keen C, Commisso J et al (1998) The effect of a marathon run on plasma and urine mineral and metal concentrations. J Am Coll Nutr 17:124–127
Burke LM (2010) Fueling strategies to optimize performance: training high or training low? Scand J Med Sci Sports 20(Suppl 2):48–58. doi:10.1111/j.1600-0838.2010.01185.x
Burke LM, Hawley JA, Wong SHS, Jeukendrup AE (2011) Carbohydrates for training and competition. J Sports Sci 29(Suppl 1):S17–S27. doi:10.1080/02640414.2011.585473
Cartee GD, Young DA, Sleeper MD et al (1989) Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol 256:E494–E499
Convertino VA, Armstrong LE, Coyle EF et al (1996) American college of sports medicine position stand. exercise and fluid replacement. Med Sci Sports Exerc 28:i–vii
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Fallon KE (2001) The acute phase response and exercise: the ultramarathon as prototype exercise. Clin J Sport Med 11:38–43
Febbraio MA, Steensberg A, Keller C et al (2003) Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J Physiol 549:607–612. doi:10.1113/jphysiol.2003.042374
Fischer CP (2006) Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev 12:6–33
Goodyear LJ, Hirshman MF, King PA et al (1990) Skeletal muscle plasma membrane glucose transport and glucose transporters after exercise. J Appl Physiol 68:193–198
Helge JW, Stallknecht B, Pedersen BK et al (2003) The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J Physiol 546:299–305
Hopkins W (2005) A spreadsheet for fully controlled crossovers. Sport Sci 9:3
Ivy JL, Katz AL, Cutler CL et al (1988) Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. J Appl Physiol 64:1480–1485
Jones AM, Doust JH (1996) A 1 % treadmill grade most accurately reflects the energetic cost of outdoor running. J Sports Sci 14:321–327. doi:10.1080/02640419608727717
Keller C, Steensberg A, Pilegaard H et al (2001) Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J 15:2748–2750. doi:10.1096/fj.01-0507fje
Kemna E, Pickkers P, Nemeth E et al (2005) Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood 106:1864–1866. doi:10.1182/blood-2005-03-1159
Kroot JJC, Laarakkers CMM, Geurts-Moespot AJ et al (2010) Immunochemical and mass-spectrometry-based serum hepcidin assays for iron metabolism disorders. Clin Chem 56:1570–1579. doi:10.1373/clinchem.2010.149187
Kroot JJC, Tjalsma H, Fleming RE, Swinkels DW (2011) Hepcidin in human iron disorders: diagnostic implications. Clin Chem 57:1650–1669. doi:10.1373/clinchem.2009.140053
Laarakkers CMM, Wiegerinck ET, Klaver S et al (2013) Improved mass spectrometry assay for plasma hepcidin: detection and characterization of a novel hepcidin isoform. PLoS One 8:e75518. doi:10.1371/journal.pone.0075518
Latunde-Dada GO (2013) Iron metabolism in athletes—achieving a gold standard. Eur J Haematol 90:10–15. doi:10.1111/ejh.12026
Lymboussaki A, Pignatti E, Montosi G et al (2003) The role of the iron responsive element in the control of ferroportin1/IREG1/MTP1 gene expression. J Hepatol 39:710–715
Nemeth E, Rivera S, Gabayan V et al (2004a) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113:1271–1276. doi:10.1172/JCI20945
Nemeth E, Tuttle MS, Powelson J et al (2004b) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093. doi:10.1126/science.1104742
Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR et al (1998) Influence of mode and carbohydrate on the cytokine response to heavy exertion. Med Sci Sports Exerc 30:671–678
Nieman DC, Davis JM, Henson DA et al (2003) Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol 94:1917–1925. doi:10.1152/japplphysiol.01130.2002
Pattini A, Schena F, Guidi GC (1990) Serum ferritin and serum iron changes after cross-country and roller ski endurance races. Eur J Appl Physiol Occup Physiol 61:55–60
Peeling P, Dawson B, Goodman C et al (2008) Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. Eur J Appl Physiol 103:381–391. doi:10.1007/s00421-008-0726-6
Peeling P, Dawson B, Goodman C et al (2009a) Effects of exercise on hepcidin response and iron metabolism during recovery. Int J Sport Nutr Exerc Metab 19:583–597
Peeling P, Dawson B, Goodman C et al (2009b) Cumulative effects of consecutive running sessions on hemolysis, inflammation and hepcidin activity. Eur J Appl Physiol 106:51–59. doi:10.1007/s00421-009-0988-7
Peeling P, Dawson B, Goodman C et al (2009c) Training surface and intensity: inflammation, hemolysis, and hepcidin expression. Med Sci Sports Exerc 41:1138–1145. doi:10.1249/MSS.0b013e318192ce58
Peeling P, Sim M, Badenhorst CE et al (2014) Iron status and the acute post-exercise hepcidin response in athletes. PLoS One 9:e93002. doi:10.1371/journal.pone.0093002
Robson-Ansley PJ, Blannin A, Gleeson M (2007) Elevated plasma interleukin-6 levels in trained male triathletes following an acute period of intense interval training. Eur J Appl Physiol 99:353–360. doi:10.1007/s00421-006-0354-y
Robson-Ansley P, Walshe I, Ward D (2011) The effect of carbohydrate ingestion on plasma interleukin-6, hepcidin and iron concentrations following prolonged exercise. Cytokine 53:196–200. doi:10.1016/j.cyto.2010.10.001
Roecker L, Meier-Buttermilch R, Brechtel L et al (2005) Iron-regulatory protein hepcidin is increased in female athletes after a marathon. Eur J Appl Physiol 95:569–571. doi:10.1007/s00421-005-0055-y
Ronsen O, Lea T, Bahr R, Pedersen BK (2002) Enhanced plasma IL-6 and IL-1ra responses to repeated vs. single bouts of prolonged cycling in elite athletes. J Appl Physiol 92:2547–2553. doi:10.1152/japplphysiol.01263.2001
Sim M, Dawson B, Landers G et al (2012) The effects of carbohydrate ingestion during endurance running on post-exercise inflammation and hepcidin levels. Eur J Appl Physiol 112:1889–1898. doi:10.1007/s00421-011-2156-0
Sim M, Dawson B, Landers GJ et al (2014) A seven day running training period increases basal urinary hepcidin levels as compared to cycling. J Int Soc Sports Nutr 11:14. doi:10.1186/1550-2783-11-14
Steensberg A, van Hall G, Osada T et al (2000) Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529(Pt 1):237–242
Steensberg A, Febbraio MA, Osada T et al (2001) Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol 537:633–639
Weight LM, Byrne MJ, Jacobs P (1991) Haemolytic effects of exercise. Clin Sci (Lond) 81:147–152
Yeo WK, Paton CD, Garnham AP et al (2008) Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J Appl Physiol 105:1462–1470. doi:10.1152/japplphysiol.90882.2008
Acknowledgments
The authors wish to acknowledge the participants who volunteered their time for this study.
Conflict of interest
The authors report no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Fabio Fischetti.
Rights and permissions
About this article
Cite this article
Badenhorst, C.E., Dawson, B., Cox, G.R. et al. Timing of post-exercise carbohydrate ingestion: influence on IL-6 and hepcidin responses. Eur J Appl Physiol 115, 2215–2222 (2015). https://doi.org/10.1007/s00421-015-3202-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-015-3202-0