Abstract
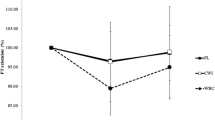
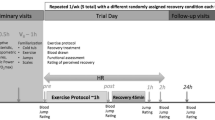
The purpose of this study was to determine the effect of cryotherapy on the inflammatory response to muscle-damaging exercise using a randomized trial. Twenty recreationally active males completed a 40-min run at a −10 % grade to induce muscle damage. Ten of the subjects were immersed in a 5 °C ice bath for 20 min and the other ten served as controls. Knee extensor peak torque, soreness rating, and thigh circumference were obtained pre- and post-run, and 1, 6, 24, 48, and 72 h post-run. Blood samples were obtained pre- and post-run, and 1, 6 and 24 h post-run for assay of plasma chemokine ligand 2 (CCL2). Peak torque decreased from 270 ± 57 Nm at baseline to 253 ± 65 Nm post-run and increased to 295 ± 68 Nm by 72 h post-run with no differences between groups (p = 0.491). Soreness rating increased from 3.6 ± 6.0 mm out of 100 mm at baseline to 47.4 ± 28.2 mm post-run and remained elevated at all time points with no differences between groups (p = 0.696). CCL2 concentrations increased from 116 ± 31 pg mL−1 at baseline to 293 ± 109 pg mL−1 at 6 h post-run (control) and from 100 ± 27 pg mL−1 at baseline to 208 ± 71 pg mL−1 at 6 h post-run (cryotherapy). The difference between groups was not significant (p = 0.116), but there was a trend for lower CCL2 in the cryotherapy group at 6 h (p = 0.102), though this measure was highly variable. In conclusion, 20 min of cryotherapy was ineffective in attenuating the strength decrement and soreness seen after muscle-damaging exercise, but may have mitigated the rise in plasma CCL2 concentration. These results do not support the use of cryotherapy during recovery.



Similar content being viewed by others
Abbreviations
- CCL2:
-
Plasma chemokine ligand 2
- DOMS:
-
Delayed-onset muscle soreness
- MVC:
-
Maximal voluntary contraction
- VASS:
-
Visual analog scale for soreness
References
Barnett A (2006) Using recovery modalities between training sessions in elite athletes: does it help? Sports Med 36(9):781–796
Best TM, Hunter KD (2000) Muscle injury and repair. Phys Med Rehabil Clin N Am 11(2):251–266
Best TM, Fiebig R, Corr DT, Brickson S, Ji L (1999) Free radical activity, antioxidant enzyme, and glutathione changes with muscle stretch injury in rabbits. J Appl Physiol 87(1):74–82
Bleakley CM, Davison GW (2010) What is the biochemical and physiological rationale for using cold-water immersion in sports recovery? A systematic review. Br J Sports Med 44(3):179–187
Brickson S, Hollander J, Corr DT, Ji LL, Best TM (2001) Oxidant production and immune response after stretch injury in skeletal muscle. Med Sci Sports Exerc 33(12):2010–2015
Brunelli S, Rovere-Querini P (2008) The immune system and the repair of skeletal muscle. Pharmacol Res 58(2):117–121
Buford TW, Cooke MB, Shelmadine BD, Hudson GM, Redd L, Willoughby DS (2009) Effects of eccentric treadmill exercise on inflammatory gene expression in human skeletal muscle. Appl Physiol Nutr Metab 34(4):745–753
Butterfield TA, Best TM, Merrick MA (2006) The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train 41(4):457–465
Camus G, Deby-Dupont G, Deby C, Juchmes-Ferir A, Pincemail J, Lamy M (1993) Inflammatory response to strenuous muscular exercise in man. Mediators Inflamm 2(5):335–342
Chazaud B, Brigitte M, Yacoub-Youssef H, Arnold L, Gherardi R, Sonnet C, Lafuste P, Chretien F (2009) Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev 37(1):18–22
Cheung K, Hume P, Maxwell L (2003) Delayed onset muscle soreness: treatment strategies and performance factors. Sports Med 33(2):145–164
Connolly DA, Sayers SP, McHugh MP (2003) Treatment and prevention of delayed onset muscle soreness. J Strength Cond Res 17(1):197–208
Eston R, Peters D (1999) Effects of cold water immersion on the symptoms of exercise-induced muscle damage. J Sports Sci 17(3):231–238
Giraldo E, Garcia JJ, Hinchado MD, Ortega E (2009) Exercise intensity-dependent changes in the inflammatory response in sedentary women: role of neuroendocrine parameters in the neutrophil phagocytic process and the pro-/anti-inflammatory cytokine balance. NeuroImmunoModulation 16(4):237–244
Howatson G, Goodall S, van Someren KA (2009) The influence of cold water immersions on adaptation following a single bout of damaging exercise. Eur J Appl Physiol 105(4):615–621
Hubal MJ, Chen TC, Thompson PD, Clarkson PM (2008) Inflammatory gene changes associated with the repeated-bout effect. Am J Physiol Regul Integr Comp Physiol 294(5):R1628–R1637
Ingram J, Dawson B, Goodman C, Wallman K, Beilby J (2009) Effect of water immersion methods on post-exercise recovery from simulated team sport exercise. J Sci Med Sport 12(3):417–421
Jakeman JR, Macrae R, Eston R (2009) A single 10-min bout of cold-water immersion therapy after strenuous polymetric exercise has no beneficial effect on recovery from the symptoms of exercise-induced muscle damage. Ergonomics 52(4):456–460
Lane KN, Wenger HA (2004) Effect of selected recovery conditions on performance of repeated bouts of intermittent cycling separated by 24 hours. J Strength Cond Res 18(4):855–860
Lapointe BM, Frenette J, Cote CH (2002) Lengthening contraction-induced inflammation is linked to secondary damage but devoid of neutrophil invasion. J Appl Physiol 92(5):1995–2004
LaRoche DP, Connolly D (2006) Effects of stretching on passive muscle tension and response to eccentric exercise. Am J Sport Med 34(6):1000–1007
LaRoche DP, Roy SJ, Knight CA, Dickie JL (2008) Elderly women have blunted response to resistance training despite reduced antagonist coactivation. Med Sci Sports Exerc 40(9):1660–1668
Lu H, Huang D, Ransohoff RM, Zhou L (2011) Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. Faseb J 25(10):3344–55 (Epub 2011 Jun 22)
Malm C, Nyberg P, Engstrom M, Sjodin B, Lenkei R, Ekblom B, Lundberg I (2000) Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol 529(Pt 1):243–262
Malm C, Sjodin TL, Sjoberg B, Lenkei R, Renstrom P, Lundberg IE, Ekblom B (2004) Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J Physiol 556(Pt 3):983–1000
Moreno LA, Joyanes M, Mesana MI, Gonzalez-Gross M, Gil CM, Sarria A, Gutierrez A, Garaulet M, Perez-Prieto R, Bueno M, Marcos A, AVENA Study Group (2003) Harmonization of anthropometric measurements for a multicenter nutrition survey in Spanish adolescents. Nutrition 19(6):481–486
Paddon-Jones DJ, Quigley BM (1997) Effect of cryotherapy on muscle soreness and strength following eccentric exercise. Int J Sports Med 18(8):588–593
Peake JM, Suzuki K, Hordem M, Wilson G, Nosaka K, Coombes JS (2005a) Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur J Appl Physiol 95:514–521
Peake J, Nosaka K, Suzuki K (2005b) Characterization of inflammatory responses to eccentric exercise in humans. Exerc Immunol Rev 11:64–85
Price DD, McGrath PA, Rafii A, Buckingham B (1983) The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 17(1):45–56
Rowsell GJ, Coutts AJ, Reaburn P, Hill-Haas S (2009) Effects of cold-water immersion on physical performance between successive matches in high-performance junior male soccer players. J Sports Sci 27(6):565–573
Sellwood KL, Brukner P, Williams D, Nicol A, Hinman R (2007) Ice-water immersion and delayed-onset muscle soreness: a randomised controlled trial. Br J Sports Med 41(6):392–397
Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JE, McManus LM (2006) MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol 81:775–785
Smith LL, McKune AJ, Semple SJ, Sibanda E, Steel H, Anderson R (2007) Changes in serum cytokines after repeated bouts of downhill running. Appl Physiol Nutr Metab 32(2):233–240
Tidball JG (2005) Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288(2):R345–R353
Tidball JG, Wehling-Henricks M (2007) Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol 578(Pt 1):327–336
Vaile J, Halson S, Gill N, Dawson B (2008) Effect of hydrotherapy on recovery from fatigue. Int J Sports Med 29(7):539–544
Yamane M, Teruya H, Nakano M, Ogai R, Ohnishi N, Kosaka M (2006) Post-exercise leg and forearm flexor muscle cooling in humans attenuates endurance and resistance training effects on muscle performance and on circulatory adaptation. Eur J Appl Physiol 96(5):572–580
Acknowledgments
The authors would like to thank the subjects who participated in this study. This study was conducted with no external funding.
Conflict of interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by William J. Kraemer.
Rights and permissions
About this article
Cite this article
Crystal, N.J., Townson, D.H., Cook, S.B. et al. Effect of cryotherapy on muscle recovery and inflammation following a bout of damaging exercise. Eur J Appl Physiol 113, 2577–2586 (2013). https://doi.org/10.1007/s00421-013-2693-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-013-2693-9