Abstract
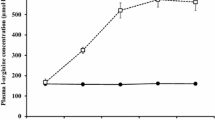
Acute tyrosine administration is associated with increased exercise capacity in the heat. To explore whether reduced plasma tyrosine and phenylalanine (tyrosine precursor) is associated with impaired exercise capacity in the heat, eight healthy, moderately trained male volunteers, unacclimated to exercise in the heat, performed two tests in a crossover design separated by at least 7 days. In a randomised, double-blind fashion, subjects ingested 500 mL flavoured, sugar-free water containing amino acids [(TYR-free; isoleucine 15 g, leucine 22.5 g, valine 17.5 g, lysine 17.5 g, methionine 5 g, threonine 10 g, tryptophan 2.5 g)] to lower the ratio of plasma tyrosine plus phenylalanine:amino acids competing for blood–brain barrier uptake (CAA), a key determinant of brain uptake, or a balanced mixture (BAL; TYR-free plus 12.5 g tyrosine and 12.5 g phenylalanine). One hour later, subjects cycled to exhaustion at 63 ± 5 % \(\dot {V}\)O2peak in 30 °C and 60 % relative humidity. Pre-exercise ratio of plasma tyrosine plus phenylalanine:ΣCAA declined 75 ± 5 % from rest in TYR-free (P < 0.001), but was unchanged in BAL (P = 0.061). Exercise time was shorter in TYR-free (59.8 ± 19.0 min vs. 66.2 ± 16.9 min in TYR-free and BAL respectively; P = 0.036). Heart rate (P = 0.298), core (P = 0.134) and skin (P = 0.384) temperature, RPE (P > 0.05) and thermal sensation (P > 0.05) were similar at exhaustion in both trials. These data indicate that acutely depleting plasma catecholamine precursors:ΣCAA is associated with reduced submaximal exercise capacity in the heat.





Similar content being viewed by others
References
Banderet LE, Lieberman HR (1989) Treatment with tyrosine, a neurotransmitter precursor, reduces environmental stress in humans. Brain Res Bull 22:759–762
Beaver WL, Wasserman K, Whipp BJ (1986) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60:2020–2027
Biggio G, Porceddu ML, Gessa GL (1976) Decrease of homovanillic, dihydroxyphenylacetic acid and cyclic-adenosine-3′,5′-monophosphate content in the rat caudate nucleus induced by the acute administration of an aminoacid mixture lacking tyrosine and phenylalanine. J Neurochem 26:1253–1255
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Bridge MW, Weller AS, Rayson M, Jones DA (2003) Responses to exercise in the heat related to measures of hypothalamic serotonergic and dopaminergic function. Eur J Appl Physiol 89:451–459
Burton A (1935) Human Calorimetry II. The average temperature of the tissues of the body. J Nutr 9:261–280
Carpenter LL, Anderson GM, Pelton GH, Gudin JA, Kirwin PD, Price LH, Heninger GR, McDougle CJ (1998) Tryptophan depletion during continuous CSF sampling in healthy human subjects. Neuropsychopharmacology 19:26–35
Cheung SS, McLellan TM (1998) Heat acclimation, aerobic fitness, and hydration effects on tolerance during uncompensable heat stress. J Appl Physiol 84:1731–1739
Cheuvront SN, Carter R 3rd, Kolka MA, Lieberman HR, Kellogg MD, Sawka MN (2004) Branched-chain amino acid supplementation and human performance when hypohydrated in the heat. J Appl Physiol 97:1275–1282
Chinevere TD, Sawyer RD, Creer AR, Conlee RK, Parcell AC (2002) Effects of l-tyrosine and carbohydrate ingestion on endurance exercise performance. J Appl Physiol 93:1590–1597
Davis JM, Bailey SP (1997) Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc 29:45–57
Dill DB, Costill DL (1974) Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37:247–248
Dollins AB, Krock LP, Storm WF, Wurtman RJ, Lieberman HR (1995) l-tyrosine ameliorates some effects of lower body negative pressure stress. Physiol Behav 57:223–230
Felig P (1975) Amino acid metabolism in man. Annu Rev Biochem 44:933–955
Fernstrom JD, Faller DV (1978) Neutral amino acids in the brain: changes in response to food ingestion. J Neurochem 30:1531–1538
Galloway SD, Maughan RJ (1997) Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc 29:1240–1249
Gijsman HJ, Scarnà A, Harmer CJ, McTavish SB, Odontiadis J, Cowen PJ, Goodwin GM (2002) A dose-finding study on the effects of branch chain amino acids on surrogate markers of brain dopamine function. Psychopharmacology 160:192–197
Gonzalez-Alonso J, Crandall CG, Johnson JM (2008) The cardiovascular challenge of exercising in the heat. J Physiol 586:45–53
Grevet EH, Tietzmann MR, Shansis FM, Hastenpflugl C, Santana LC, Forster L, Kapczinskil F, Izquierdo I (2002) Behavioural effects of acute phenylalanine and tyrosine depletion in healthy male volunteers. J Psychopharmacol 16:51–55
Kenefick RW, Cheuvront SN, Palombo LJ, Ely BR, Sawka MN (2010) Skin temperature modifies the impact of hypohydration on aerobic performance. J Appl Physiol 109:79–86
Lehnert H, Reinstein DK, Strowbridge BW, Wurtman RJ (1984) Neurochemical and behavioral consequences of acute, uncontrollable stress: effects of dietary tyrosine. Brain Res 303:215–223
Leyton M, Young SN, Blier P, Baker GB, Pihl RO, Benkelfat C (2000a) Acute tyrosine depletion and alcohol ingestion in healthy women. Alcohol Clin Exp Res 24:459–464
Leyton M, Young SN, Pihl RO, Etezadi S, Lauze C, Blier P, Baker GB, Benkelfat C (2000b) Effects on mood of acute phenylalanine/tyrosine depletion in healthy women. Neuropsychopharmacology 22:52–63
Lieberman H (1994) Tyrosine and stress: animal and human studies. In: Marriott B (ed) Food components to enhance performance. National Academy Press, Washington DC, pp 277–299
Lieberman HR, Georgelis JH, Maher TJ, Yeghiayan SK (2005) Tyrosine prevents effects of hyperthermia on behavior and increases norepinephrine. Physiol Behav 84:33–38
Magill RA, Waters WF, Bray GA, Volaufova J, Smith SR, Lieberman HR, McNevin N, Ryan DH (2003) Effects of tyrosine, phentermine, caffeine D-amphetamine, and placebo on cognitive and motor performance deficits during sleep deprivation. Nutr Neurosci 6:237–246
Mahoney CR, Castellani J, Kramer FM, Young A, Lieberman HR (2007) Tyrosine supplementation mitigates working memory decrements during cold exposure. Physiol Behav 92:575–582
Masurier ML, Oldenzeil W, Lehman C, Cowen P, Sharp T (2006) Effect of acute tyrosine depletion in using a branched chain amino-acid on dopamine neurotransmission in the rat brain. Neuropsychopharmacology 31:310–317
McTavish SF, Cowen PJ, Sharp T (1999a) Effect of a tyrosine-free amino acid mixture on regional brain catecholamine synthesis and release. Psychopharmacology 141:182–188
McTavish SF, McPherson MH, Sharp T, Cowen PJ (1999b) Attenuation of some subjective effects of amphetamine following tyrosine depletion. J Psychopharmacol 13:144–147
McTavish SF, McPherson MH, Harmer CJ, Clark L, Sharp T, Goodwin GM, Cowen PJ (2001) Antidopaminergic effects of dietary tyrosine depletion in healthy subjects and patients with manic illness. Br J Psychiatry 179:356–360
McTavish SF, Mannie ZN, Harmer CJ, Cowen PJ (2005) Lack of effect of tyrosine depletion on mood in recovered depressed women. Neuropsychopharmacology 30:786–791
Moja EA, Lucini V, Benedetti F, Lucca A (1996) Decrease in plasma phenylalanine and tyrosine after phenylalanine-tyrosine free amino acid solutions in man. Life Sci 58:2389–2395
Montgomery AJ, McTavish SF, Cowen PJ, Grasby PM (2003) Reduction of brain dopamine concentration with dietary tyrosine plus phenylalanine depletion: an [11C]raclopride PET study. Am J Psychiatry 160:1887–1889
Nielsen B, Savard G, Richter EA, Hargreaves M, Saltin B (1990) Muscle blood flow and muscle metabolism during exercise and heat stress. J Appl Physiol 69:1040–1046
Nielsen B, Hyldig T, Bidstrup F, González-Alonso J, Christoffersen GR (2001) Brain activity and fatigue during prolonged exercise in the heat. Pflugers Arch 442:41–48
Nybo L, Nielsen B (2001a) Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol 91:1055–1060
Nybo L, Nielsen B (2001b) Perceived exertion is associated with an altered brain activity during exercise with progressive hyperthermia. J Appl Physiol 91:2017–2023
Nybo L, Secher NH, Nielsen B (2002) Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol 545:697–704
Pardridge WM (1983) Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev 63:1481–1535
Parkin JM, Carey MF, Zhao S, Febbraio MA (1999) Effect of ambient temperature on human skeletal muscle metabolism during fatiguing submaximal exercise. J Appl Physiol 86:902–908
Parsons K (2003) Human thermal environments. Taylor and Francis, London
Perronet F, Massicote D (1991) Table of non-protein respiratory quotient: an update. Can J Sports Sci 16:23–29
Ramanathan NL (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19:531–533
Robinson OJ, Standing HR, DeVito EE, Cools R, Sahakian BJ (2010) Dopamine precursor depletion improves punishment prediction during reversal learning in healthy females but not males. Psychopharmacology 211:187–195
Roiser JP, McLean A, Ogilvie AD, Blackwell AD, Bamber DJ, Goodyer I, Jones PB, Sahakian BJ (2005) The subjective and cognitive effects of acute phenylalanine and tyrosine depletion in patients recovered from depression. Neuropsychopharmacology 30:775–785
Sawka MN, Latzka WA, Matott RP, Montain SJ (1998) Hydration effects on temperature regulation. Int J Sports Med 19(Suppl 2):S108–S110
Sheehan B, Tharyan P, McTavish S, Campling G, Cowen P (1996) Use of a dietary manipulation to deplete plasma tyrosine and phenylalanine in healthy subjects. J Psychopharmacol 10:231–234
Shurtleff D, Thomas JR, Schrot J, Kowalski K, Harford R (1994) Tyrosine reverses a cold-induced working memory deficit in humans. Pharmacol Biochem Behav 47:935–941
Strüder HK, Hollmann W, Platen P, Donike M, Gotzmann A, Weber K (1998) Influence of paroxetine, branched-chain amino acids and tyrosine on neuroendocrine system responses and fatigue in humans. Horm Metab Res 30:188–194
Tumilty L, Davison G, Beckmann M, Thatcher R (2011) Oral tyrosine supplementation improves exercise capacity in the heat. Eur J Appl Physiol 111:2941–2950
Watson P, Shirreffs SM, Maughan RJ (2004) The effect of acute branched-chain amino acid supplementation on prolonged exercise capacity in a warm environment. Eur J Appl Physiol 93:306–314
Watson P, Hasegawa H, Roelands B, Piacentini MF, Looverie R, Meeusen R (2005) Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol 565:873–883
Watson P, Enever S, Page A, Stockwell J, Maughan RJ (2012) Tyrosine supplementation does not influence the capacity to perform prolonged exercise in a warm environment. Int J Sports Nutr Ex Metab 22:363–373
Williams WA, Shoaf SE, Hommer D, Rawlings R, Linnoila M (1999) Effects of acute tryptophan depletion on plasma and cerebrospinal fluid tryptophan and 5-hydroxyindoleacetic acid in normal volunteers. J Neurochem 72:1641–1647
Yeghiayan SK, Luo S, Shukitt-Hale B, Lieberman HR (2001) Tyrosine improves behavioral and neurochemical deficits caused by cold exposure. Physiol Behav 72:311–316
Acknowledgments
The authors would like to thank SHS international for their kind donation of amino acid powders for use in this study.
Conflict of interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by George Havenith.
Rights and permissions
About this article
Cite this article
Tumilty, L., Davison, G., Beckmann, M. et al. Acute oral administration of a tyrosine and phenylalanine-free amino acid mixture reduces exercise capacity in the heat. Eur J Appl Physiol 113, 1511–1522 (2013). https://doi.org/10.1007/s00421-012-2577-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2577-4