Abstract
Continuous positive airway pressure (CPAP) is a treatment modality for pulmonary oxygenation difficulties. CPAP impairs venous return to the heart and, in turn, affects cerebral blood flow (CBF) and augments cerebral blood volume (CBV). We considered that during CPAP, elevation of the upper body would prevent a rise in CBV, while orthostasis would challenge CBF. To determine the body position least affecting indices of CBF and CBV, the middle cerebral artery mean blood velocity (MCA V mean) and the near-infrared spectroscopy determined frontal cerebral hemoglobin content (cHbT) were evaluated in 11 healthy subjects during CPAP at different body positions (15° head-down tilt, supine, 15°, 30° and 45° upper body elevation). In the supine position, 10 cmH2O of CPAP reduced MCA V mean by 9 ± 3% and increased cHbT by 4 ± 2 μmol/L (mean ± SEM); (P < 0.05). In the head-down position, CPAP increased cHbT to 13 ± 2 μmol/L but left MCA V mean unchanged. Upper body elevation by 15° attenuated the CPAP associated reduction in MCA V mean (−7 ± 2%), while cHbT returned to baseline (1 ± 2 μmol/L). With larger elevation of the upper body MCA V mean decreased progressively to −17 ± 3%, while cHbT remained unchanged from baseline. These results suggest that upper body elevation by ∼15° during 10 cmH2O CPAP prevents an increase in cerebral blood volume with minimal effect on cerebral blood flow.
Similar content being viewed by others
Introduction
Continuous positive airway pressure (CPAP) and upper body elevation are applied for treatment of respiratory insufficiency because they improve pulmonary oxygenation by increasing pulmonary functional residual capacity (Sevransky et al. 2004). In the post-operative setting, CPAP is used for alveolar recruitment and/or for the prevention of atelectasis (Pinilla et al. 1990; Ricksten et al. 1986) and in patients with obstructive sleep apnoea nasal CPAP is applied to for prevention of upper airway obstruction (Mansfield et al. 2004). Another means of improving pulmonary function and preventing ventilator-associated pneumonia in patients is a backrest elevation (Dellinger et al. 2004; Drakulovic et al. 1999). A semi recumbent position also improves upper airway stability in patients with obstructive sleep apnoea (Neill et al. 1997).
Both CPAP and upper body elevation affect cerebral hemodynamics. In supine humans, cerebral arterial inflow and venous outflow pressures are similar to the corresponding pressures at the level of the heart. Thus, elevation of the intrathoracic pressure by CPAP impairs venous return to the heart and, in turn, affects cerebral blood flow (CBF) and augments cerebral blood volume (CBV) (Pott et al. 2000). With progressive elevation of the upper body, the neck veins collapse (Dawson et al. 2004; Gisolf et al. 2004; Valdueza et al. 2000) thus protecting the brain from an increased intrathoracic pressure (Toung et al. 2000). In neuro-intensive care, the backrest is elevated ∼30° to prevent a gravitational increase in CBV and thus intracranial pressure (ICP) (Fan 2004). On the other hand, upper body elevation reduces the central blood volume and decreases arterial pressure at the level of the brain. During CPAP, blood flow velocity in the middle cerebral artery (MCA V mean) is reported to increase (Haring et al. 1994), to decrease (Kolbitsch et al. 2000; Scala et al. 2003), or to remain unaffected (Bowie et al. 2001; Droste et al. 1999). These discrepancies appear to relate to different degrees of head elevation and control of the arterial carbon dioxide (CO2) tension among the studies. Therefore, the purpose of this study was to determine the body position least affecting indices of CBF and CBV, the MCA V mean and the near-infrared determined total frontal cerebral hemoglobin concentrations (cHbT) in healthy subjects during CPAP at different upper body positions.
Methods
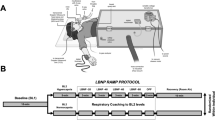
Eleven healthy volunteers (1 woman), aged 25 years (20–32; mean and range), height 183 cm (169–190); weight 79 kg (65–102) participated in this investigation. All subjects provided written informed consent prior to the study as approved by the Ethics Committee for Copenhagen and Frederiksberg (KF 01 287338).
The subjects were allowed a light breakfast on the day of the experiment with no restrictions on requirements of fluid intake. Changes in cerebral concentrations of oxygenated (cHbO2) and deoxygenated hemoglobin (cHb) were assessed by a NIRO 500 near-infrared spectroscopy (NIRS) apparatus (Hamamatsu Photonics Corp., Osaka, Japan). The light source and the sensing optode were fastened to the left forehead and covered with a dark cloth for light shielding. Changes in the total cerebral hemoglobin concentration (cHbT) were the sum of cHbO2 and cHb. The proximal segment of the right MCA was insonated at a depth of 50–54 mm through the “temporal window” using a Doppler apparatus (DWL, Sipplingen, Germany). After the best signal-to-noise ratio was established, the probe was fastened to the head with adhesive ultrasonic gel (Tensive, Parker Laboratories Inc., Fairfield, NJ, USA) and secured using a custom-made headband. A soft plastic mask (VBM Medizintechnik GmbH, Sulz, Germany) was fitted over the subject’s nose and mouth using elastic bands. CPAP was applied using a Whisperflow fixed flow generator using pressurized room air and Whisperflow isobaric CPAP valves at 5 and 10 cmH2O opening pressure (Caradyne, Galway, Ireland). No valves were used in the first positional cycle. A pressure manometer verified the pressure retainment of the system throughout the respiratory cycle. The end-tidal carbon dioxide tension (PetCO2) was measured (Datex-Ohmeda Inc., Madison, WI, USA) and finger arterial pressure was measured with a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands). The cuff was applied to the midphalanx of the middle finger of the dominant hand and placed at heart level.
Instrumentation occurred at 9 a.m. in a room at 22°C and thereafter, the subjects were made to lie supine in a hospital bed and allowed to rest for 10 min. In order to account for changes in MCA V mean due to changes in the arterial CO2 tension, the subjects performed a CO2-reactivity test. During normoventilation in the supine position, PetCO2 and MCA V mean were measured, followed by two periods of 30 s with moderate voluntary hyperventilation aiming to decrease PetCO2 by 1 and 2 kPa, respectively. Following 4 min of supine rest, the backrest was elevated by 15°, 30° and 45°, and, finally, the subjects were in a 15° head-down position; with each position maintained for 4 min. The same sequence of events was repeated with CPAP of 5 and 10 cmH2O. All variables were A/D converted and sampled at 100 Hz, 16 bit, on PCI-Base 1000 hardware and NextView software (BMC Messsysteme GmbH, Berlin, Germany).
Data analysis
The MCA velocity traces were inspected offline and artifacts were removed using MATLAB 7.1 analysis software (MathWorks, Natick, MA, USA). The finger arterial pressure curve was analyzed using Beatscope software (Finapres Medical). Beat-to-beat systolic, diastolic and mean arterial (MAP) pressures, as well as stroke volume (SV) were computed from the arterial pressure pulse wave by off-line Modelflow analysis. This method computes an aortic flow waveform by simulating a nonlinear, time-varying model of the aortic input impedance, thereby calculating SV reliably (Harms et al. 1999). Cardiac output (CO) was SV times heart rate (HR) and total peripheral resistance (TPR) was the ratio of mean arterial pressure (MAP) to CO. For each upper body position, blood pressure at the level of the MCA (MAPMCA) was calculated as MAP at the level of the heart minus the hydrostatic difference. MCA beat-to-beat systolic, diastolic and mean flow velocities were determined from the outline curve of the transcranial Doppler spectrum using Beatscope software. NIRS data were normalized to baseline, defined as values from the sample interval in the supine position without CPAP. All variables were transformed to equidistantly resampled data at 1 Hz by polynomial interpolation and averaged over 30 s prior to the end of each intervention.
For calculation of MCA V mean − CO2-reactivity, the last 15 s of baseline and the two periods of hyperventilation were obtained. The CO2-reactivity was calculated using polynomial interpolation, assuming a linear relationship between PetCO2 and MCA V mean for the range of PetCO2 investigated. In order to assess whether changes in MCA V mean were determined by changes in the CO2 tension, the individual CO2-reactivity was used to adjust MCA V mean values to the baseline PetCO2.
Statistics
Data are expressed as mean ± SEM unless otherwise indicated. Changes over time were examined by Friedman’s repeated measures analysis of variance on ranks. Changes between interventions were examined with the Student–Newman–Keul’s test and a P < 0.05 was considered statistically significant.
Results
Effects of CPAP
In the supine position 5 cmH2O CPAP had no effect on MCA V mean, or cHbT, while cHbO2 increased 2 ± 2 μmol/L (Fig. 1). MAPMCA, HR, SV, and CO remained unchanged (Fig. 2). Increasing CPAP to 10 cmH2O diminished MCA V mean by 9 ± 3% from baseline, while cHbT increased 4 ± 2 μmol/L and cHbO2 increased further to 5 ± 2 μmol/L. SV decreased by 3 ± 3% from baseline with MAPMCA unchanged (Fig. 2). PetCO2 tended to decline with increasing levels of CPAP without reaching statistical significance (Fig. 1).
Cerebral and ventilatory responses to continuous positive airway pressure (CPAP) at different body positions. Changes in middle cerebral artery mean blood velocity (ΔMCA V mean), changes in frontal cerebral total, oxygenated and deoxygenated hemoglobin concentrations (ΔcHbT, ΔcHbO2, ΔcHb), and end-tidal carbon dioxide tension (PetCO2) during CPAP 0 cmH2O (circles), 5 cmH2O (squares), and 10 cmH2O (triangles), respectively. Filled symbols, different from baseline (supine, CPAP 0 cmH2O) P < 0.05. * Different from CPAP 0 cmH2O at the same body position (CPAP effect), P < 0.05. # Different from supine at the same level of CPAP (positional effect), P < 0.05
Circulatory responses to continuous positive airway pressure (CPAP) at different body positions. Changes in mean arterial pressure at brain level (MAP MCA ), total peripheral resistance (TPR), cardiac stroke volume (SV), heart rate (HR), and cardiac output (CO) during CPAP 0 cmH2O (circles), 5 cmH2O (squares), and 10 cmH2O (triangles), respectively. Filled symbols, different from baseline (supine, CPAP 0 cmH2O) P < 0.05. * Different from CPAP 0 cmH2O at the same body position (CPAP effect), P < 0.05. # Different from supine at the same level of CPAP (positional effect), P < 0.05
Effects of upper body elevation
With increasing levels of upper body elevation, MCA V mean decreased. The largest decrease was by 8 ± 2% from baseline at 45°; also cHbO2 decreased with upper body elevation reaching statistical significance at 45° (4 ± 2 μmol/L) while cHbT remained unchanged (Fig. 1). MAPMCA decreased 7 ± 3 mmHg from baseline at 45° even though TPR increased 107 ± 26 dyn s−1 cm−5 (∼15%). With a small increase in HR and a proportional reduction in SV (5 ± 2%), CO did not change (Fig. 2). Also PetCO2 was stable.
Effects of CPAP and upper body elevation
5 cmH2O CPAP at 45° upper body elevation decreased MCA V mean 11 ± 3% and to 17 ± 3% when increasing CPAP to 10 cmH2O. At CPAP of 0 and 5 cmH2O and regardless of the degree of upper body elevation, cHbT remained unchanged. CPAP of 10 cmH2O had no effect on cHbT as long as the upper body was elevated at least 15°. The increased cHbT with 10 cmH2O CPAP when supine, returned to baseline with 15° upper body elevation (1 ± 2 μmol/L). With both 5 and 10 cmH2O CPAP, cHbO2 decreased when the upper body was elevated 15° and did not decrease further with higher elevation (Fig. 1). In positions with the upper body elevated, SV decreased by 4.8 ± 0.8% for each 5 cmH2O increase of CPAP, reaching a nadir of −14 ± 4% at 45° with 10 cmH2O of CPAP. In the upper body elevated positions HR did not change with the application of 5 cmH2O, but increased with 10 cmH2O of CPAP. CO tended to decline when CPAP was applied, but this did not reach statistical significance (Fig. 2).
Head-down position
MCA V mean and MAPMCA remained unchanged while cHbT rose by 9 ± 1 μmol/L during the head-down position. SV and CO increased 4 ± 2% and 6 ± 2%, respectively. Adding 5 and 10 cmH2O CPAP further elevated cHbT to 11 ± 2 μmol/L and 13 ± 2 μmol/L, respectively, but had no effect on MCA V mean (Fig. 1). CPAP reduced HR and CO proportionally. MAPMCA was unaffected by 5 cmH2O of CPAP, but rose with an increase to 10 cmH2O. Compared to the corresponding supine CPAP level, in the head-down position, SV was elevated at 5 and 10 cmH2O CPAP (Fig. 2).
CO2-reactivity
The CO2-reactivity was 21 ± 2.7%/kPa (2.8 ± 0.36%/mmHg). The CO2-adjusted MCA V mean decreased with both CPAP and upper body elevation, by 12 ± 3% at 45° upper body elevation with a CPAP of 10 cmH2O (data not shown).
Discussion
This study investigated the influence of upper body position on MCA V mean and cHbT during continuous positive airway pressure breathing. In the supine position and especially in the head down position, CPAP elevated cHbT indicating impaired cerebrovenous drainage. This effect was eliminated by upper body elevation. On the other hand, CPAP did not affect MCA V mean in the head-down position, but provoked a decrease in the supine position and with upper body elevation to 30° and 45°. As indicated by a stable cHbT and an only minor reduction in MCA V mean, 15° upper body elevation balances the effect of 10 cmH2O of CPAP on augmentation of CBV and that of orthostasis on a reduction in CBF.
To appreciate these conclusions it needs to be addressed that transcranial Doppler monitors blood flow velocity rather than volume flow, and changes in the diameter of the insonated vessel could modulate velocity independently of flow. During craniotomy, the diameter of the MCA remains unchanged by even large changes in arterial pressure (Giller 1989). Furthermore, as determined with magnetic resonance imaging during changes in PetCO2 and in simulated orthostasis, the diameter of the MCA remains stable, suggesting that the MCA is not involved in regulation of cerebral vascular resistance (Serrador et al. 2000). These results suggest that changes in MCA V mean reflect those in cerebral blood flow.
A NIRS apparatus was used to evaluate changes in the hemoglobin concentration of superficial brain tissue. ΔcHbT, i.e. the sum of changes in oxy- and deoxyhemoglin reflects changes in total hemoglobin concentration of the interrogated tissue and was taken to indicate changes in cerebral blood volume. In the supine and head-down positions, ΔcHbT increased with application of CPAP but such an effect could not be detected with upper body elevation suggesting a hydrostatic influence counteracting the CPAP-induced cerebrovenous congestion. With NIRS equipment similar to that applied in our study, ΔcHbT is reported to correlate with the level of expiratory pressure and the magnitude of change is similar to that found in our study (Elwell et al. 1996). In the head-down position there was a large increase in ΔcHbT suggesting a significant increase in CBV. However, venous stasis to the skin and skull may have contributed to changes in light attenuation (Firbank et al. 1998; Rostrup et al. 2002), while an influence from the tissues surrounding the brain is considered of minor importance in the supine and head elevated positions (Owen-Reece et al. 1996).
Changes in posture and CPAP affect the central blood volume (Secher and Van Lieshout 2005) and, in turn, stroke volume of the heart (Leonetti et al. 2004). In the supine position gravity does not compromise the central blood volume and only a CPAP of 10 cmH2O caused a reduction in SV. With upper body elevation even minor reductions in preload as imposed by CPAP of 5 cmH2O, diminished SV, unmasking the reduced central blood volume. In the head down position no decrease in SV occurred with CPAP, indicating that the heart is not preload limited, i.e., a “surplus” preload exists, large enough to accept the reduction by CPAP. An increase in SV from supine to the head down position has been reported in healthy volunteers (McInnis et al. 2006; Shiraishi et al. 2002; Soubiran et al. 1996), but may imply that the subjects were not normovolaemic (van Lieshout et al. 2005). We consider our subjects normovolaemic as they were not fasting and had free access to fluids. Furthermore, in the supine position SV was robust to preload reduction by 5 cmH2O of CPAP. It may be speculated that the observed increase in SV upon head down positioning is facilitated by reduced afterload as TPR decreased. Both during CPAP and upper body elevation the decline in CO was not statistically significant, reflecting baroreflex control of HR that limits the effects of a reduction in SV on CO. Such compensation appears to be exhausted with larger elevation of the upper body and higher intrathoracic pressure, as demonstrated during a Valsalva manuevre (mouth pressure 40 mmHg) in the sitting position when CO decreases ∼24% (Pott et al. 2003).
Both MCA V mean and SV fell in parallel with upper body elevation and during CPAP treatment. At the same time MAP at the level of the MCA was well within the range of blood pressure where cerebral autoregulation is traditionally considered to maintain CBF stable (Lassen 1959). These observations add to evidence that cardiac output and stroke volume of the heart may influence cerebral vascular resistance and in turn blood flow to the brain independently of blood pressure (Ide et al. 1998, 1999; Immink et al. 2006; van Lieshout et al. 2001, 2003). TPR increased with both upper body elevation and CPAP and was lowest in the head-down position reflecting the level of systemic sympathetic activity (Kardos et al. 1997; Nagaya et al. 1995). Systemic sympathetic activity may also act on resistance vessels of the brain. Thus, when blockade of the stellate ganglion diminishes sympathetic discharge to brain vessels, it reverses a reduction in MCA V mean that is associated with lowered cardiac output (Ide et al. 2000). We suggest that a reduction of cerebrovascular resistance accounts for the unchanged MCA V mean in the head-down position when a reduced blood flow to the brain would be expected by impaired venous outflow. However, other factors may have contributed to influence MCA V mean, e.g., alterations in the arterial CO2 tension, MAP and/or the level of cerebral activation.
The arterial CO2 tension is a major determinant for blood flow to the brain. The PetCO2 as an indicator for arterial carbon dioxide tension remained statistically unchanged during head elevation and CPAP treatment, although a trend was noted toward a decrease. To evaluate whether individual changes in MCA V mean were dominated by changes in the arterial carbon dioxide tension, MCA V mean was adjusted for the changes in PetCO2 using the individual CO2 reactivity. This CO2-adjusted MCA V mean demonstrated a similar time course. As CPAP increases the ventilation/perfusion ratio of the lungs, PetCO2 overestimates the fall in arterial CO2 tension (Immink et al. 2006). Taken together, these findings suggest that arterial CO2 play only a minor role in regard to the drop in MCA V mean during CPAP treatment.
When supine the jugular veins are open in their entire length and jugular bulb pressure equals central venous pressure (Cirovic et al. 2003; Dawson et al. 2004). Accordingly, in the supine position 10 cmH2O of CPAP led to a decrease in MCA V mean of ∼10% while a rise in cHbT suggests augmentation of cerebral capacitance vessels and, in turn, increased outflow resistance. With upper body elevation, the jugular veins collapse and the cerebral venous outflow path shifts from the jugular to the vertebral venous system (Dawson et al. 2004; Gisolf et al. 2004; Valdueza et al. 2000). In the upright position, the jugular veins may also be reopened by positive pressure breathing (Gisolf et al. 2004). Interestingly, elevating the upper body by 15° during 10 cmH2O of CPAP, increased the MCA V mean, while HbT decreased. This was the case in spite of the changes in systemic hemodynamics that are induced by CPAP in that position. Thus, upper body elevation per se decreases cerebral blood flow through the systemic hemodynamic effects of reduced cardiac preload combined with the unfavorable position of the brain relative to the heart. Nonetheless, upper body elevation may support cerebral blood flow in the presence of cerebral venous congestion imposed by positive pressure breathing, i.e., by elevating the brain above the hydrostatic threshold for transmittance of intrathoracic pressure.
Opening of the jugular veins by positive airway pressure has been proposed a mechanism to improve cerebral perfusion by reducing jugular venous resistance to flow (Cirovic et al. 2003). An open jugular vein may be important especially for prevention of acceleration-induced loss of consciousness when gravitational forces not only displace blood to the lower extremities, but also aggravate the collapse of the jugular veins. Our results do not indicate that CPAP supports blood flow to the brain during the moderate gravitational challenge up to 45° upper body elevation. On the contrary, these results manifest the importance of venous return to the heart that is impaired both by gravity and intrathoracic pressure.
MCA V mean reached a nadir of 17% below baseline in the 45° upper body elevated position with 10 cmH2O CPAP. This reduction is similar to that observed under everyday physiological challenges, i.e., standing up (Pott et al. 2000). Although these changes appear to be of little importance for healthy subjects, the magnitude of changes in flow and, in turn, its consequences for the injured brain may be significant. In patients suffering from acute intracranial pathology the backrest of the bed is elevated ∼30°. That is the case although MAP decreases at the level of the brain (Durward et al. 1983). Usually, a positive effect of upper body elevation is found on cerebral perfusion pressure, i.e., the difference between blood pressure at the level of the brain and intracranial pressure (Fan 2004). Head elevation reduces cerebral blood flow (Moraine et al. 2000), while averaged intracerebral tissue oxygen tension remains unaffected, although the individual changes may be large (Ng et al. 2004). As indicated by our study, a negative consequence of upper body elevation for blood flow to the brain is aggravated by positive pressure ventilation. The position of the backrest may, ideally, be based on an individual flow and/or oxygenation measurement (March et al. 1990). We consider that for patients suffering from cerebral pathology and in need of pulmonary support by CPAP and upper body elevation, improvements in blood oxygenation may outweigh the negative consequences on cerebral blood flow.
As indicated by cHbT and MCA V mean, a balance between alleviating cerebral congestion and maintaining CBF during 10 cmH2O of CPAP is achieved with 15° upper body elevation. Further upper body elevation has no consequences for the cerebral blood volume but leads to a progressive decline in MCA V mean suggesting that cerebral blood flow is compromised.
References
Bowie RA, O’Connor PJ, Hardman JG, Mahajan RP (2001) The effect of continuous positive airway pressure on cerebral blood flow velocity in awake volunteers. Anesth Analg 92:415–417
Cirovic S, Walsh C, Fraser WD, Gulino A (2003) The effect of posture and positive pressure breathing on the hemodynamics of the internal jugular vein. Aviat Space Environ Med 74:125–131
Dawson EA, Secher NH, Dalsgaard MK, Ogoh S, Yoshiga CC, Gonzalez-Alonso J, Steensberg A, Raven PB (2004) Standing up to the challenge of standing: a siphon does not support cerebral blood flow in humans. Am J Physiol Regul Integr Comp Physiol 287:R911–R914
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent JL, Levy MM (2004) Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 32:858–873
Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M (1999) Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet 354:1851–1858
Droste DW, Ludemann P, Anders F, Kemeny V, Thomas M, Krauss JK, Ringelstein EB (1999) Middle cerebral artery blood flow velocity, end-tidal pCO2 and blood pressure in patients with obstructive sleep apnea and in healthy subjects during continuous positive airway pressure breathing. Neurol Res 21:737–741
Durward QJ, Amacher AL, Del Maestro RF, Sibbald WJ (1983) Cerebral and cardiovascular responses to changes in head elevation in patients with intracranial hypertension. J Neurosurg 59:938–944
Elwell CE, Owen-Reece H, Wyatt JS, Cope M, Reynolds EO, Delpy DT (1996) Influence of respiration and changes in expiratory pressure on cerebral haemoglobin concentration measured by near infrared spectroscopy. J Cereb Blood Flow Metab 16:353–357
Fan JY (2004) Effect of backrest position on intracranial pressure and cerebral perfusion pressure in individuals with brain injury: a systematic review. J Neurosci Nurs 36:278–288
Firbank M, Okada E, Delpy DT (1998) A theoretical study of the signal contribution of regions of the adult head to near-infrared spectroscopy studies of visual evoked responses. Neuroimage 8:69–78
Giller CA (1989) Transcranial Doppler monitoring of cerebral blood velocity during craniotomy. Neurosurgery 25:769–776
Gisolf J, van Lieshout JJ, van Heusden K, Pott F, Stok WJ, Karemaker JM (2004) Human cerebral venous outflow pathway depends on posture and central venous pressure. J Physiol 560:317–327
Haring HP, Hormann C, Schalow S, Benzer A (1994) Continuous positive airway pressure breathing increases cerebral blood flow velocity in humans. Anesth Analg 79:883–885
Harms MP, Wesseling KH, Pott F, Jenstrup M, van Goudoever J, Secher NH, van Lieshout JJ (1999) Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 97:291–301
Ide K, Boushel R, Sørensen HM, Fernandes A, Cai Y, Pott F, Secher NH (2000) Middle cerebral artery blood velocity during exercise with beta-1 adrenergic and unilateral stellate ganglion blockade in humans. Acta Physiol Scand 170:33–38
Ide K, Gulløv AL, Pott F, van Lieshout JJ, Koefoed BG, Petersen P, Secher NH (1999) Middle cerebral artery blood velocity during exercise in patients with atrial fibrillation. Clin Physiol 19:284–289
Ide K, Pott F, van Lieshout JJ, Secher NH (1998) Middle cerebral artery blood velocity depends on cardiac output during exercise with a large muscle mass. Acta Physiol Scand 162:13–20
Immink RV, Secher NH, Roos CM, Pott F, Madsen PL, van Lieshout JJ (2006) The postural reduction in middle cerebral artery blood velocity is not explained by PaCO2. Eur J Appl Physiol 96:609–614
Kardos A, Rudas L, Simon J, Gingl Z, Csanady M (1997) Effect of postural changes on arterial baroreflex sensitivity assessed by the spontaneous sequence method and Valsalva manoeuvre in healthy subjects. Clin Auton Res 7:143–148
Kolbitsch C, Lorenz IH, Hormann C, Schocke M, Kremser C, Zschiegner F, Felber S, Benzer A (2000) The impact of increased mean airway pressure on contrast-enhanced MRI measurement of regional cerebral blood flow (rCBF), regional cerebral blood volume (rCBV), regional mean transit time (rMTT), and regional cerebrovascular resistance (rCVR) in human volunteers. Hum Brain Mapp 11:214–222
Lassen NA (1959) Cerebral blood flow and oxygen consumption in man. Physiol Rev 39:183–238
Leonetti P, Audat F, Girard A, Laude D, Lefrere F, Elghozi JL (2004) Stroke volume monitored by modeling flow from finger arterial pressure waves mirrors blood volume withdrawn by phlebotomy. Clin Auton Res 14:176–181
Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT (2004) Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med 169:361–366
March K, Mitchell P, Grady S, Winn R (1990) Effect of backrest position on intracranial and cerebral perfusion pressures. J Neurosci Nurs 22:375–381
McInnis NH, Journeay WS, Jay O, Leclair E, Kenny GP (2006) 15 degrees head-down tilt attenuates the postexercise reduction in cutaneous vascular conductance and sweating and decreases esophageal temperature recovery time. J Appl Physiol 101:840–847
Moraine JJ, Berre J, Melot C (2000) Is cerebral perfusion pressure a major determinant of cerebral blood flow during head elevation in comatose patients with severe intracranial lesions? J Neurosurg 92:606–614
Nagaya K, Wada F, Nakamitsu S, Sagawa S, Shiraki K (1995) Responses of the circulatory system and muscle sympathetic nerve activity to head-down tilt in humans. Am J Physiol 268:R1289–R1294
Neill AM, Angus SM, Sajkov D, McEvoy RD (1997) Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 155:199–204
Ng I, Lim J, Wong HB (2004) Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery 54:593–597
Owen-Reece H, Elwell CE, Wyatt JS, Delpy DT (1996) The effect of scalp ischaemia on measurement of cerebral blood volume by near-infrared spectroscopy. Physiol Meas 17:279–286
Pinilla JC, Oleniuk FH, Tan L, Rebeyka I, Tanna N, Wilkinson A, Bharadwaj B (1990) Use of a nasal continuous positive airway pressure mask in the treatment of postoperative atelectasis in aortocoronary bypass surgery. Crit Care Med 18:836–840
Pott F, van Lieshout JJ, Ide K, Madsen P, Secher NH (2000) Middle cerebral artery blood velocity during a valsalva maneuver in the standing position. J Appl Physiol 88:1545–1550
Pott F, van Lieshout JJ, Ide K, Madsen P, Secher NH (2003) Middle cerebral artery blood velocity during intense static exercise is dominated by a Valsalva maneuver. J Appl Physiol 94:1335–1344
Ricksten SE, Bengtsson A, Soderberg C, Thorden M, Kvist H (1986) Effects of periodic positive airway pressure by mask on postoperative pulmonary function. Chest 86:774–781
Rostrup E, Law I, Pott F, Ide K, Knudsen GM (2002) Cerebral hemodynamics measured with simultaneous PET and near-infrared spectroscopy in humans. Brain Res 954:183–193
Scala R, Turkington PM, Wanklyn P, Bamford J, Elliott MW (2003) Effects of incremental levels of continuous positive airway pressure on cerebral blood flow velocity in healthy adult humans. Clin Sci (Lond) 104:633–639
Secher NH, Van Lieshout JJ (2005) Normovolaemia defined by central blood volume and venous oxygen saturation. Clin Exp Pharmacol Physiol 32:901–910
Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL (2000) MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31:1672–1678
Sevransky JE, Levy MM, Marini JJ (2004) Mechanical ventilation in sepsis-induced acute lung injury/acute respiratory distress syndrome: an evidence-based review. Crit Care Med 32:S548–S553
Shiraishi M, Schou M, Gybel M, Christensen NJ, Norsk P (2002) Comparison of acute cardiovascular responses to water immersion and head-down tilt in humans. J Appl Physiol 92:264–268
Soubiran C, Harant I, de G I, Beauville M, Crampes F, Riviere D, Garrigues M (1996) Cardio-respiratory changes during the onset of head-down tilt. Aviat Space Environ Med 67:648–653
Toung TJ, Aizawa H, Traystman RJ (2000) Effects of positive end-expiratory pressure ventilation on cerebral venous pressure with head elevation in dogs. J Appl Physiol 88:655–661
Valdueza JM, von Munster T, Hoffman O, Schreiber S, Einhaupl KM (2000) Postural dependency of the cerebral venous outflow. Lancet 355:200–201
van Lieshout JJ, Pott F, Madsen PL, van Goudoever J, Secher NH (2001) Muscle tensing during standing: effects on cerebral tissue oxygenation and cerebral artery blood velocity. Stroke 32:1546–1551
van Lieshout JJ, Wieling W, Karemaker JM, Secher NH (2003) Syncope, cerebral perfusion, and oxygenation. J Appl Physiol 94:833–848
van Lieshout JJ, Harms MP, Pott F, Jenstrup M, Secher NH (2005) Stroke volume of the heart and thoracic fluid content during head-up and head-down tilt in humans. Acta Anaesthesiol Scand 49:1287–1292
Acknowledgments
We are grateful to the staff at the Department of Clinical Physiology, Bispebjerg Hospital for lending us equipment for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Højlund Rasmussen, J., Mantoni, T., Belhage, B. et al. Influence of upper body position on middle cerebral artery blood velocity during continuous positive airway pressure breathing. Eur J Appl Physiol 101, 369–375 (2007). https://doi.org/10.1007/s00421-007-0513-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-007-0513-9