Abstract
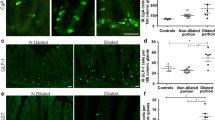
Patients suffering from chagasic megacolon must have an intact mucosal barrier as they survive this chronic disease for decades. A key structure of the mucosal barrier are epithelial cells. Vasoactive-intestinal-peptide (VIP)-positive nerve fibres are involved in influencing, e.g., epithelial cell proliferation, mucus secretion (e.g., mucin 2 and trefoil factor 3 of goblet cells) and inflammation or autoimmunity, all putative and/or known factors altered in chagasic megacolon. We analyzed qualitatively and quantitatively goblet cells, their specific markers, such as mucin 2 (MUC2) and trefoil factor 3 (TFF3) and enterocytes, the relation of VIP-immunoreactive nerve fibres to the epithelia, the distribution of gelsolin, a protein involved in chronic inflammation processes in the epithelia, and the proliferation rate of epithelial cells by combined 4′,6-diamidino-2-phenylindole (DAPI) and phosphohistone-H3 (PHH3) staining. Goblet cells were the dominating epithelial cell type. They accounted for 38.4% of all epithelial cells in controls and changed to 58.9% in the megacolonic parts. In contrast to the overall expression in goblet cells of control epithelia, TFF3 was confined to goblet cells at the base of the crypts whereas MUC2 was found only in luminal goblet cells. Gelsolin-positive goblet cells were predominantly recognized within the controls. Finally, the mean value of mitosis increased from 1.5% within the controls up to 2.6% in the anal parts of the chagasic sepcimens. Taken together, increased cell proliferation, preponderance of goblet cells, differential MUC 2, and TFF 3 expression might all be factors maintaining an intact mucosal barrier within chagasic megacolon.





Similar content being viewed by others
References
Adad SJ, Etchebehere RM, Araujo JR, Madureira AB, Lima VG, Silva AA, Eduardo C (2002) Association of chagasic megacolon and cancer of the colon: case report and review of the literature. Rev Soc Bras Med Trop 35(1):63–68
Ben-Horin S, Chowers Y (2008) Neuroimmunology of the gut: physiology, pathology, and pharmacology. Curr Opin Pharmacol 8(4):490–495. doi:10.1016/j.coph.2008.07.010
Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, Vallance BA (2010) Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog 6(5):e1000902. doi:10.1371/journal.ppat.1000902
Boyer L, Sidpra D, Jevon G, Buchan AM, Jacobson K (2007) Differential responses of VIPergic and nitrergic neurons in paediatric patients with Crohn’s disease. Auton Neurosci 134 (1–2):106–114. doi:10.1016/j.autneu.2007.03.001
Chagas C (1909) Nova tripanozomiaze humana. Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n.gen. n.sp., ajente etiolojico de nova entidade morbida do homem. Mem Inst Oswaldo Cruz 1:159–218. doi:10.1590/S0074-02761909000200008
da Silveira AB, Lemos EM, Adad SJ, Correa-Oliveira R, Furness JB, D’Avila Reis D (2007) Megacolon in Chagas disease: a study of inflammatory cells, enteric nerves, and glial cells. Hum Pathol 38(8):1256–1264. doi:10.1016/j.humpath.2007.01.020
Dutra WO, Menezes CA, Villani FN, da Costa GC, da Silveira AB, Reis D, Gollob KJ (2009) Cellular and genetic mechanisms involved in the generation of protective and pathogenic immune responses in human Chagas disease. Mem Inst Oswaldo Cruz 104(Suppl 1):208–218
Garcia S (1995) O câncer no megacólon: estudos da incidência no homem e experimental em ratos. Tese de Doutorado.. Ribeirão Preto. Universidade de São Paulo, SP
Garcia SB, Oliveira JS, Pinto LZ, Muccillo G, Zucoloto S (1996) The relationship between megacolon and carcinoma of the colon: an experimental approach. Carcinogenesis 17(8):1777–1779
Garcia SB, Stopper H, Kannen V (2014) The contribution of neuronal-glial-endothelial-epithelial interactions to colon carcinogenesis. Cell Mol Life Sci 71(17):3191–3197. doi:10.1007/s00018-014-1642-z
Gersemann M, Becker S, Kubler I, Koslowski M, Wang G, Herrlinger KR, Griger J, Fritz P, Fellermann K, Schwab M, Wehkamp J, Stange EF (2009) Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation 77(1):84–94. doi:10.1016/j.diff.2008.09.008
Henry M, Mendes E, Prado R, Saad L, Gonçalves-Júnior I, Macedo A (1989) Megacólon: análise de 200 pacientes submetidos a tratamento cirúrgico. Revista Goiana de Medicina 35:25–33
Hokari R, Lee H, Crawley SC, Yang SC, Gum JR Jr, Miura S, Kim YS (2005) Vasoactive intestinal peptide upregulates MUC2 intestinal mucin via CREB/ATF1. Am J Physiol Gastrointest Liver Physiol 289(5):G949–G959. doi:10.1152/ajpgi.00142.2005
Hudson L, Hindmarsh PJ (1985) The relationship between autoimmunity and Chagas’ disease: causal or coincidental? Curr Top Microbiol Immunol 117:167–177
Jabari S, da Silveira AB, de Oliveira EC, Neto SG, Quint K, Neuhuber W, Brehmer A (2011) Partial, selective survival of nitrergic neurons in chagasic megacolon. Histochem Cell Biol 135(1):47–57. doi:10.1007/s00418-010-0774-y
Jabari S, da Silveira AB, de Oliveira EC, Quint K, Wirries A, Neuhuber W, Brehmer A (2013) Interstitial cells of Cajal: crucial for the development of megacolon in human Chagas’ disease? Colorectal Dis 15(10):e592–e598. doi:10.1111/codi.12331
Jabari S, da Silveira AB, de Oliveira EC, Quint K, Wirries A, Neuhuber W, Brehmer A (2014a) Mucosal layers and related nerve fibres in non-chagasic and chagasic human colon–a quantitative immunohistochemical study. Cell Tissue Res 358(1):75–83. doi:10.1007/s00441-014-1934-5
Jabari S, de Oliveira EC, Brehmer A, da Silveira AB (2014b) Chagasic megacolon: enteric neurons and related structures. Histochem Cell Biol 142(3):235–244. doi:10.1007/s00418-014-1250-x
Johansson ME, Hansson GC (2013) Mucus and the goblet cell. Dig Dis 31(3–4):305–309. doi:10.1159/000354683
Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC (2008) The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 105(39):15064–15069. doi:10.1073/pnas.0803124105
Johansson ME, Sjovall H, Hansson GC (2013) The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 10(6):352–361. doi:10.1038/nrgastro.2013.35
Kannen V, de Oliveira EC, Motta BZ, Chaguri AJ, Brunaldi MO, Garcia SB (2015) Trypanosomiasis-induced megacolon illustrates how myenteric neurons modulate the risk for colon cancer in rats and humans. PLoS Negl Trop Dis 9(4):e0003744. doi:10.1371/journal.pntd.0003744
Kim YS, Ho SB (2010) Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep 12(5):319–330. doi:10.1007/s11894-010-0131-2
Koberle F (1968) Chagas’ disease and Chagas’ syndromes: the pathology of American trypanosomiasis. Adv Parasitol 6:63–116
Kwiatkowski DJ (1999) Functions of gelsolin: motility, signaling, apoptosis, cancer. Curr Opin Cell Biol 11(1):103–108
Leow CC, Romero MS, Ross S, Polakis P, Gao WQ (2004) Hath1, down-regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res 64(17):6050–6057. doi:10.1158/0008-5472.CAN-04-0290
Li GH, Arora PD, Chen Y, McCulloch CA, Liu P (2012) Multifunctional roles of gelsolin in health and diseases. Med Res Rev 32(5):999–1025. doi:10.1002/med.20231
Li WX, Yang MX, Hong XQ, Dong TG, Yi T, Lin SL, Qin XY, Niu WX (2016) Overexpression of gelsolin reduces the proliferation and invasion of colon carcinoma cells. Mol Med Rep 14 (4):3059–3065. doi:10.3892/mmr.2016.5652
Meneses A, Lopes M, Rocha A, Fatureto M, Lopes G, Lopes E, Chapadeiro E (1989) Megas e câncer. Câncer de intestino grosso em chagásicos com megacólon. Arquivos de Gastroenterologia 26:13–16
Moro F, Levenez F, Durual S, Plaisancie P, Thim L, Giraud AS, Cuber JC (2001) Secretion of the trefoil factor TFF3 from the isolated vascularly perfused rat colon. Regul Pept 101(1–3):35–41
Mulisch MW, Ulrich (2010) Romeis - Mikroskopische Technik. Springer Spektrum. doi:10.1007/978-3-8274-2254-5
Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A (2003) Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol 285(5):G1028–G1036. doi:10.1152/ajpgi.00066.2003
Neunlist M, Van Landeghem L, Mahe MM, Derkinderen P, des Varannes SB, Rolli-Derkinderen M (2013) The digestive neuronal-glial-epithelial unit: a new actor in gut health and disease. Nat Rev Gastroenterol Hepatol 10(2):90–100. doi:10.1038/nrgastro.2012.221
Ogata H, Podolsky DK (1997) Trefoil peptide expression and secretion is regulated by neuropeptides and acetylcholine. The American journal of physiology 273(2 Pt 1):G348–G354
Oliveira E (1998) Associação entre infecção crônica pelo Trypanosoma cruzi e câncer de cólon. Estudo experimental em ratos. Tese de Mestrado. Universidade Federal de Goiás, Goiânia
Oliveira EC, Leite MS, Miranda JA, Andrade AL, Garcia SB, Luquetti AO, Moreira H (2001) Chronic Trypanosoma cruzi infection associated with low incidence of 1,2-dimethylhydrazine-induced colon cancer in rats. Carcinogenesis 22(5):737–740
Pelaseyed T, Bergstrom JH, Gustafsson JK, Ermund A, Birchenough GM, Schutte A, van der Post S, Svensson F, Rodriguez-Pineiro AM, Nystrom EE, Wising C, Johansson ME, Hansson GC (2014) The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev 260(1):8–20. doi:10.1111/imr.12182
Rassi A Jr, Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375(9723):1388–1402. doi:10.1016/S0140-6736(10)60061-X
Ribalta T, McCutcheon IE, Aldape KD, Bruner JM, Fuller GN (2004) The mitosis-specific antibody anti-phosphohistone-H3 (PHH3) facilitates rapid reliable grading of meningiomas according to WHO 2000 criteria. Am J Surg Pathol 28(11):1532–1536
Rocha A, Almeida H, Esper F, Moraes D, Santos E, Teixeira V, (1983) A associação entre megaesôjago e carcinoma de esôjago. Revista da Sociedade Brasileira de Medicina Tropical 16:94–97
Strugala V, Dettmar PW, Pearson JP (2008) Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn’s disease. Int J Clin Pract 62(5):762–769. doi:10.1111/j.1742-1241.2007.01665.x
Wu X, Conlin VS, Morampudi V, Ryz NR, Nasser Y, Bhinder G, Bergstrom KS, Yu HB, Waterhouse CC, Buchan AM, Popescu OE, Gibson WT, Waschek JA, Vallance BA, Jacobson K (2015) Vasoactive intestinal polypeptide promotes intestinal barrier homeostasis and protection against colitis in mice. PloS one 10(5):e0125225. doi:10.1371/journal.pone.0125225
Yamaguchi H, Condeelis J (2007) Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta 1773(5):642–652. doi:10.1016/j.bbamcr.2006.07.001
Acknowledgements
The present work was performed in fulfillment of the requirements of the Friedrich-Alexander University of Erlangen-Nürnberg for obtaining the degree “Dr. med.” of C.K.. The excellent technical assistance of Karin Löschner, Stefanie Link, Anita Hecht, Andrea Hilpert, and Hedwig Symowski is gratefully acknowledged. This study was supported by Dr. Ernst und Anita Bauer-Stiftung and Luise Prell-Stiftung.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Koch, C., da Silveira, A.B.M., de Oliveira, E.C. et al. Epithelial cell types and their proposed roles in maintaining the mucosal barrier in human chagasic–megacolonic mucosa. Histochem Cell Biol 148, 207–216 (2017). https://doi.org/10.1007/s00418-017-1563-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-017-1563-7