Abstract
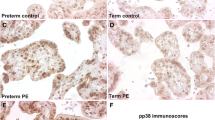
To evaluate the expression of markers correlated with cellular senescence and DNA damage (8-hydroxy-2′-deoxy-guanosine (8-OHdG), p53, p21, APE1/Ref-1 (APE1), interleukin (IL-6 and IL-8) in placentas from healthy and pathologic pregnancies. This retrospective study considered a placental tissue micro-array containing 92 controls from different gestational ages and 158 pathological cases including preeclampsia (PE), HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count), small for gestational age (SGA) fetuses, and intrauterine growth restriction (IUGR) occurring at different gestational ages. In this study, we demonstrated a significant influence of gestational age on the expression in the trophoblast of 8-OHdG, p53, p21, APE1, and IL-6. In placentas of cases affected by PE, HELLP, or IUGR, there was an increased expression of 8-OHdG, p53, APE1, and IL-6 compared to controls (only IL-8 was significantly decreased in cases). In both groups of pathology between 22- and 34-week gestation and after 34-week gestation, APE1 levels were higher in the trophoblast of women affected by hypertensive disorders of pregnancy than women carrying an IUGR fetus. The cytoplasmic expression of 8-OHdG was increased in placentas in IUGR cases compared to PE or HELLP pregnancies. In cases after 34-week gestation, p21 was higher in SGA and IUGR than in controls and late PE. Moreover, p53 was increased after 34-week gestation in IUGR pregnancies. Placentas from pathological pregnancies had an altered expression of 8-OHdG, p53, p21, APE1, IL-6, and IL-8. The alterations of intracellular pathways involving these elements may be the cause or the consequence of placental dysfunction, but in any case reflect an impaired placental function, possibly due to increased aging velocity in pathologic cases.



Similar content being viewed by others
Abbreviations
- 8-OHdG:
-
8-Hydroxy-2′-deoxy-guanosine
- AP:
-
Apurinic/apyrimidinic
- APE1:
-
APE1/Ref-1 (apurinic apyrimidinic endonuclease redox effector factor-1)
- BER:
-
Base excision repair
- BMI:
-
Body mass index
- DNA:
-
Deoxyribonucleic acid
- HELLP:
-
Hemolysis, elevated liver enzymes, low platelet count
- IL-6:
-
Interleukin-6
- IL-8:
-
Interleukin-8
- IUGR:
-
Intrauterine growth restriction
- mRNA:
-
Messenger ribonucleic acid
- PE:
-
Preeclampsia
- RNA:
-
Ribonucleic acid
- SASP:
-
Senescence-associated secretory phenotype
- SGA:
-
Small for gestational age
References
Amu S, Hahn-Zoric M, Malik A, Ashraf R, Zaman S, Kjellmer I, Hagberg H, Padyukov L, Hanson LA (2006) Cytokines in the placenta of Pakistani newborns with and without intrauterine growth retardation. Pediatr Res 59:254–258. doi:10.1203/01.pdr.0000196332.37565.7d
Barchiesi A, Wasilewski M, Chacinska A, Tell G, Vascotto C (2015) Mitochondrial translocation of ape1 relies on the mia pathway. Nucleic Acids Res 43:5451–5464. doi:10.1093/nar/gkv433
Baxter JK, Weinstein L (2004) Hellp syndrome: the state of the art. Obstet Gynecol Surv 59:838–845
Biron-Shental T, Sukenik-Halevy R, Sharon Y, Goldberg-Bittman L, Kidron D, Fejgin MD, Amiel A (2010) Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol 202:381.e1–387.e7. doi:10.1016/j.ajog.2010.01.036
Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM (2001) The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the international society for the study of hypertension in pregnancy (isshp). Hypertens Pregnancy 20:IX–XIV. doi:10.1081/PRG-100104165
Burton DGA, Krizhanovsky V (2014) Physiological and pathological consequences of cellular senescence. Cell Mol Life Sci 71:4373–4386. doi:10.1007/s00018-014-1691-3
Cesaratto L, Codarin E, Vascotto C, Leonardi A, Kelley MR, Tiribelli C, Tell G (2013) Specific inhibition of the redox activity of ape1/ref-1 by e3330 blocks tnf-α-induced activation of il-8 production in liver cancer cell lines. PLoS One 8:e70909. doi:10.1371/journal.pone.0070909
Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic ras and the p53 tumor suppressor. PLoS Biol 6:2853–2868. doi:10.1371/journal.pbio.0060301
Costa F, Panagodage S, Brennecke S, Murthi P (2013) Oc03.01: low-dose aspirin improves trophoblastic function in early-onset pre-eclampsia. Ultrasound Obstet Gynecol 42:5. doi:10.1002/uog.12591
Davy P, Nagata M, Bullard P, Fogelson NS, Allsopp R (2009) Fetal growth restriction is associated with accelerated telomere shortening and increased expression of cell senescence markers in the placenta. Placenta 30:539–542. doi:10.1016/j.placenta.2009.03.005
Endo H, Okamoto A, Yamada K, Nikaido T, Tanaka T (2005) Frequent apoptosis in placental villi from pregnancies complicated with intrauterine growth restriction and without maternal symptoms. Int J Mol Med 16:79–84
Ewen ME, Miller SJ (1996) p53 and translational control. Biochim Biophys Acta 1242:181–184
Fruscalzo A, Schmitz R, Klockenbusch W, Köhler G, Londero AP, Siwetz M, Huppertz B (2012) Human placental transthyretin in fetal growth restriction in combination with preeclampsia and the hellp syndrome. Histochem Cell Biol 138:925–932. doi:10.1007/s00418-012-0997-1
Fujimaki A, Watanabe K, Mori T, Kimura C, Shinohara K, Wakatsuki A (2011) Placental oxidative dna damage and its repair in preeclamptic women with fetal growth restriction. Placenta 32:367–372. doi:10.1016/j.placenta.2011.02.004
Ginsberg D, Mechta F, Yaniv M, Oren M (1991) Wild-type p53 can down-modulate the activity of various promoters. Proc Natl Acad Sci USA 88:9979–9983
Hahn-Zoric M, Hagberg H, Kjellmer I, Ellis J, Wennergren M, Hanson LA (2002) Aberrations in placental cytokine mrna related to intrauterine growth retardation. Pediatr Res 51:201–206. doi:10.1203/00006450-200202000-00013
Hayakawa T, Iwai M, Aoki S, Takimoto K, Maruyama M, Maruyama W, Motoyama N (2015) Sirt1 suppresses the senescence-associated secretory phenotype through epigenetic gene regulation. PLoS One 10:e0116480. doi:10.1371/journal.pone.0116480
Heazell AEP, Lacey HA, Jones CJP, Huppertz B, Baker PN, Crocker IP (2008) Effects of oxygen on cell turnover and expression of regulators of apoptosis in human placental trophoblast. Placenta 29:175–186. doi:10.1016/j.placenta.2007.11.002
Heazell AEP, Sharp AN, Baker PN, Crocker IP (2011) Intra-uterine growth restriction is associated with increased apoptosis and altered expression of proteins in the p53 pathway in villous trophoblast. Apoptosis 16:135–144. doi:10.1007/s10495-010-0551-3
Izutsu T, Kudo T, Sato T, Nishiya I, Ohyashiki K, Mori M, Nakagawara K (1998) Telomerase activity in human chorionic villi and placenta determined by trap and in situ trap assay. Placenta 19:613–618
Jeschke U, Schiessl B, Mylonas I, Kunze S, Kuhn C, Schulze S, Friese K, Mayr D (2006) Expression of the proliferation marker ki-67 and of p53 tumor protein in trophoblastic tissue of preeclamptic, hellp, and intrauterine growth-restricted pregnancies. Int J Gynecol Pathol 25:354–360. doi:10.1097/01.pgp.0000225838.29127.6
Jones CJ, Fox H (1980) An ultrastructural and ultrahistochemical study of the human placenta in maternal pre-eclampsia. Placenta 1:61–76
Ju Z, Choudhury AR, Rudolph KL (2007) A dual role of p21 in stem cell aging. Ann NYAcad Sci 1100:333–344. doi:10.1196/annals.1395.036
Kimura C, Watanabe K, Iwasaki A, Mori T, Matsushita H, Shinohara K, Wakatsuki A (2013) The severity of hypoxic changes and oxidative dna damage in the placenta of early-onset preeclamptic women and fetal growth restriction. J Matern Fetal Neonatal Med 26:491–496. doi:10.3109/14767058.2012.733766
Kudo T, Izutsu T, Sato T (2000) Telomerase activity and apoptosis as indicators of ageing in placenta with and without intrauterine growth retardation. Placenta 21:493–500. doi:10.1053/plac.2000.0538
Levy R, Smith SD, Yusuf K, Huettner PC, Kraus FT, Sadovsky Y, Nelson DM (2002) Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am J Obstet Gynecol 186:1056–1061
Ljungman M (2000) Dial 9-1-1 for p53: mechanisms of p53 activation by cellular stress. Neoplasia 2:208–225
Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW (2005) Dna repair, genome stability, and aging. Cell 120:497–512. doi:10.1016/j.cell.2005.01.028
Londero AP, Bertozzi S, Visentin S, Fruscalzo A, Driul L, Marchesoni D (2013) High placental index and poor pregnancy outcomes: a retrospective study of 18 386 pregnancies. Gynecol Endocrinol 29:666–669. doi:10.3109/09513590.2013.798273
Madlener S, Ströbel T, Vose S, Saydam O, Price BD, Demple B, Saydam N (2013) Essential role for mammalian apurinic/apyrimidinic (ap) endonuclease ape1/ref-1 in telomere maintenance. Proc Natl Acad Sci USA 110:17844–17849. doi:10.1073/pnas.1304784110
Miyashita T, Kitada S, Krajewski S, Horne WA, Delia D, Reed JC (1995) Overexpression of the bcl-2 protein increases the half-life of p21bax. J Biol Chem 270:26049–26052
Reed M, Woelker B, Wang P, Wang Y, Anderson ME, Tegtmeyer P (1995) The c-terminal domain of p53 recognizes dna damaged by ionizing radiation. Proc Natl Acad Sci USA 92:9455–9459
Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C (1998) Bcl-2 prolongs cell survival after bax-induced release of cytochrome c. Nature 391:496–499. doi:10.1038/35160
Rossi DJ, Jamieson CHM, Weissman IL (2008) Stems cells and the pathways to aging and cancer. Cell 132:681–696. doi:10.1016/j.cell.2008.01.036
Rossi A, Bortolotti N, Vescovo S, Romanello I, Forzano L, Londero AP, Ambrosini G, Marchesoni D, Curcio F (2013) Ischemia-modified albumin in pregnancy. Eur J Obstet Gynecol Reprod Biol. doi:10.1016/j.ejogrb.2013.06.037
Sargent IL, Borzychowski AM, Redman CWG (2006) Nk cells and human pregnancy—an inflammatory view. Trends Immunol 27:399–404. doi:10.1016/j.it.2006.06.009
Takagi Y, Nikaido T, Toki T, Kita N, Kanai M, Ashida T, Ohira S, Konishi I (2004) Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch 444:49–55. doi:10.1007/s00428-003-0903-2
Tamura D, Merideth M, DiGiovanna JJ, Zhou X, Tucker MA, Goldstein AM, Brooks BP, Khan SG, Oh KS, Ueda T, Boyle J, Moslehi R, Kraemer KH (2011) High-risk pregnancy and neonatal complications in the dna repair and transcription disorder trichothiodystrophy: report of 27 affected pregnancies. Prenat Diagn 31:1046–1053. doi:10.1002/pd.2829
Tell G, Damante G, Caldwell D, Kelley MR (2005) The intracellular localization of ape1/ref-1: more than a passive phenomenon? Antioxid Redox Signal 7:367–384. doi:10.1089/ars.2005.7.367
Tell G, Wilson DM 3rd, Lee CH (2010) Intrusion of a dna repair protein in the rnome world: is this the beginning of a new era? Mol Cell Biol 30:366–371. doi:10.1128/MCB.01174-09
Thakur S, Sarkar B, Cholia RP, Gautam N, Dhiman M, Mantha AK (2014) Ape1/ref-1 as an emerging therapeutic target for various human diseases: phytochemical modulation of its functions. Exp Mol Med 46:e106. doi:10.1038/emm.2014.42
Thakur S, Dhiman M, Tell G, Mantha AK (2015) A review on protein-protein interaction network of ape1/ref-1 and its associated biological functions. Cell Biochem Funct 33:101–112. doi:10.1002/cbf.3100
Vascotto C, Salzano AM, D’Ambrosio C, Fruscalzo A, Marchesoni D, di Loreto C, Scaloni A, Tell G, Quadrifoglio F (2007) Oxidized transthyretin in amniotic fluid as an early marker of preeclampsia. J Proteome Res 6:160–170. doi:10.1021/pr060315z
Vascotto C, Fantini D, Romanello M, Cesaratto L, Deganuto M, Leonardi A, Radicella JP, Kelley MR, D’Ambrosio C, Scaloni A, Quadrifoglio F, Tell G (2009) Ape1/ref-1 interacts with npm1 within nucleoli and plays a role in the rrna quality control process. Mol Cell Biol 29:1834–1854. doi:10.1128/MCB.01337-08
Visentin S, Lapolla A, Londero AP, Cosma C, Dalfrà M, Camerin M, Faggian D, Plebani M, Cosmi E (2014) Adiponectin levels are reduced while markers of systemic inflammation and aortic remodelling are increased in intrauterine growth restricted mother-child couple. Biomed Res Int. doi:10.1155/2014/401595
Wang Y, Walsh SW (1998) Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 19:581–586
Young ARJ, Narita M (2009) Sasp reflects senescence. EMBO Rep 10:228–230. doi:10.1038/embor.2009.22
Acknowledgments
We are grateful to Eilidh PJ McIntosh for her suggestions on the style and composition of our English. We are grateful to Vito D’Aietti for his precious help. We are grateful to Matteo De Luca for the technical assistance in realizing TMA and Marta Forgiarini and Magdalena Marciniak for technical support. We are also grateful to Prof. Diego Marchesoni, Prof. Carlo Alberto Beltrami, Prof. Carla Di Loreto, Prof. Dr. med. Walter Klockenbusch, and Prof. Gabriele Köhler for their help and suggestions. Furthermore, this research is based on the Ph.D. dissertation of Dr Ambrogio P Londero that took place in the University of Udine.
Authors’ contribution
APL, MO, TG, SM, AF, SB, NN, ES, and LM made substantial contributions to conception and design or acquisition of data or to analysis and interpretation of data. APL, MO, AC, SM, AF, SB, NN, LD, GT, RJL, and LM were involved in drafting the article or revising it critically for important intellectual content. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no potential conflicts of interest relevant to this article. This study had no financial support.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Londero, A.P., Orsaria, M., Marzinotto, S. et al. Placental aging and oxidation damage in a tissue micro-array model: an immunohistochemistry study. Histochem Cell Biol 146, 191–204 (2016). https://doi.org/10.1007/s00418-016-1435-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-016-1435-6