Abstract
Purpose
Intracellular formation of advanced glycation end products (AGEs) is a crucial pathological process in retinal diseases such as age-related macular degeneration (AMD) or diabetic retinopathy (DR). Glyoxal is a physiological metabolite produced during formation of AGEs and has also been shown to derive from photodegraded bisretinoid fluorophores in aging retinal pigment epithelial (RPE) cells.
Methods
Flow cytometry was combined with either: 1) immunocytochemical staining to detect glyoxal induced formation of Nε-carboxymethyllysine (CML)-modifications of intracellular proteins (AGEs) and changes in the production of stress response proteins; or 2) vital staining to determine apoptosis rates (annexin V binding), formation of intracellular reactive oxygen species (ROS), mitochondrial membrane potential (MMP), and changes in intracellular pH upon treatment of cells with glyoxal. The percentage of apoptotic cells was further quantified by flow cytometry after staining of fixed cells with propidium iodide to determine cells with a subdiploid (fragmented) DNA content. Apoptosis related activation of caspase 3 was determined by Western blotting. Glyoxal induced changes in VEGF-A165a mRNA expression and protein production were determined by real-time PCR and by flow cytometry after immunocytochemical staining.
Results
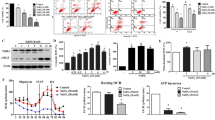
Increasing glyoxal concentrations resulted in enhanced formation of AGEs, such as CML modifications of proteins. This was associated with elevated levels of intracellular reactive oxygen species, a depolarized MMP, and a decreased intracellular pH, resulting in an increased number of apoptotic cells. Apoptosis related caspase 3 activation increased in a dose dependent manner after glyoxal incubation. In consequence, the cells activated compensatory mechanisms and increased the levels of the anti-oxidative and stress-related proteins heme oxygenase-1, osteopontin, heat shock protein 27, copper/zinc superoxide dismutase, manganese superoxide dismutase, and cathepsin D. Furthermore, VEGF-A165a mRNA expression and VEGF-A protein production were significantly increased after incubation with glyoxal in ARPE-19 cells.
Conclusions
The glyoxal-induced oxidative stress and apoptosis in ARPE-19 cells may provide a suitable in vitro model for studying RPE cellular reactions to AGEs that occur in AMD or in DR.






Similar content being viewed by others
References
Stitt AW (2001) Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br J Ophthalmol 85:746–753
Tian J, Ishibashi K, Ishibashi K, Reiser K, Grebe R, Biswal S, Gehlbach P, Handa JT (2005) Advanced glycation endproduct-induced aging of the retinal pigment epithelium and choroid: a comprehensive transcriptional response. Proc Natl Acad Sci U S A 102:11846–11851
Milne R, Brownstein S (2013) Advanced glycation end products and diabetic retinopathy. Amino Acids 44:1397–1407
Munch G, Thome J, Foley P, Schinzel R, Riederer P (1997) Advanced glycation endproducts in ageing and Alzheimer’s disease. Brain Res Brain Res Rev 23:134–143
Sensi M, Pricci F, Andreani D, Di Mario U (1991) Advanced nonenzymatic glycation endproducts (AGE): their relevance to aging and the pathogenesis of late diabetic complications. Diabetes Res 16:1–9
Ulrich P, Cerami A (2001) Protein glycation, diabetes, and aging. Recent Prog Horm Res 56:1–21
Ishibashi T, Murata T, Hangai M, Nagai R, Horiuchi S, Lopez PF, Hinton DR, Ryan SJ (1998) Advanced glycation end products in age-related macular degeneration. Arch Ophthalmol 116:1629–1632
Farboud B, Aotaki-Keen A, Miyata T, Hjelmeland LM, Handa JT (1999) Development of a polyclonal antibody with broad epitope specificity for advanced glycation endproducts and localization of these epitopes in Bruch’s membrane of the aging eye. Mol Vis 5:11
Hammes HP, Hoerauf H, Alt A, Schleicher E, Clausen JT, Bretzel RG, Laqua H (1999) N(epsilon)(carboxymethyl)lysin and the AGE receptor RAGE colocalize in age-related macular degeneration. Invest Ophthalmol Vis Sci 40:1855–1859
Schmidt AM, Yan SD, Yan SF, Stern DM (2001) The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest 108:949–955
Abordo EA, Minhas HS, Thornalley PJ (1999) Accumulation of alpha-oxoaldehydes during oxidative stress: a role in cytotoxicity. Biochem Pharmacol 58:641–648
Thornalley PJ, Langborg A, Minhas HS (1999) Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J 344(Pt 1):109–116
Morgan PE, Dean RT, Davies MJ (2002) Inactivation of cellular enzymes by carbonyls and protein-bound glycation/glycoxidation products. Arch Biochem Biophys 403:259–269
Stitt AW (2003) The role of advanced glycation in the pathogenesis of diabetic retinopathy. Exp Mol Pathol 75:95–108
Shapiro R, Cohen BI, Shiuey SJ, Maurer H (1969) On the reaction of guanine with glyoxal, pyruvaldehyde, and kethoxal, and the structure of the acylguanines. A new synthesis of N2-alkylguanines. Biochemistry 8:238–245
Lederer MO, Klaiber RG (1999) Cross-linking of proteins by Maillard processes: characterization and detection of lysine-arginine cross-links derived from glyoxal and methylglyoxal. Bioorg Med Chem 7:2499–2507
Chetyrkin S, Mathis M, Pedchenko V, Sanchez OA, McDonald WH, Hachey DL, Madu H, Stec D, Hudson B, Voziyan P (2011) Glucose autoxidation induces functional damage to proteins via modification of critical arginine residues. Biochemistry 50:6102–6112
Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR (1996) The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem 271:9982–9986
Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW (1995) Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry 34:3702–3709
Yoon KD, Yamamoto K, Ueda K, Zhou J, Sparrow JR (2012) A novel source of methylglyoxal and glyoxal in retina: implications for age-related macular degeneration. PLoS One 7:e41309
Jaattela M (1999) Heat shock proteins as cellular lifeguards. Ann Med 31:261–271
Milbury PE, Graf B, Curran-Celentano JM, Blumberg JB (2007) Bilberry (Vaccinium myrtillus) anthocyanins modulate heme oxygenase-1 and glutathione S-transferase-pi expression in ARPE-19 cells. Invest Ophthalmol Vis Sci 48:2343–2349
Kutty RK, Nagineni CN, Kutty G, Hooks JJ, Chader GJ, Wiggert B (1994) Increased expression of heme oxygenase-1 in human retinal pigment epithelial cells by transforming growth factor-beta. J Cell Physiol 159:371–378
Sumi D, Ignarro LJ (2004) Regulation of inducible nitric oxide synthase expression in advanced glycation end product-stimulated raw 264.7 cells: the role of heme oxygenase-1 and endogenous nitric oxide. Diabetes 53:1841–1850
Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM (1996) ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res 62:155–169
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139:271–279
Jakubowski W, Bartosz G (2000) 2,7-dichlorofluorescin oxidation and reactive oxygen species: what does it measure? Cell Biol Int 24:757–760
Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB (1995) Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol 260:406–417
Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A (1997) JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett 411:77–82
Nuydens R, Novalbos J, Dispersyn G, Weber C, Borgers M, Geerts H (1999) A rapid method for the evaluation of compounds with mitochondria-protective properties. J Neurosci Methods 92:153–159
Franck P, Petitipain N, Cherlet M, Dardennes M, Maachi F, Schutz B, Poisson L, Nabet P (1996) Measurement of intracellular pH in cultured cells by flow cytometry with BCECF-AM. J Biotechnol 46:187–195
Bird AC (2003) The Bowman lecture. Towards an understanding of age-related macular disease. Eye 17:457–466
Zarbin MA (2004) Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 122:598–614
Ahsan H (2015) Diabetic retinopathy--biomolecules and multiple pathophysiology. Diabetes Metab Syndr 9:51–54
Fleming T, Nawroth PP (2014) Reactive metabolites as a cause of late diabetic complications. Biochem Soc Trans 42:439–442
Hidmark A, Fleming T, Vittas S, Mendler M, Deshpande D, Groener JB, Muller BP, Reeh PW, Sauer SK, Pham M, Muckenthaler MU, Bendszus M, Nawroth PP (2014) A new paradigm to understand and treat diabetic neuropathy. Exp Clin Endocrinol Diabetes 122:201–207
Fleming T, Cuny J, Nawroth G, Djuric Z, Humpert PM, Zeier M, Bierhaus A, Nawroth PP (2012) Is diabetes an acquired disorder of reactive glucose metabolites and their intermediates? Diabetologia 55:1151–1155
Han Y, Randell E, Vasdev S, Gill V, Gadag V, Newhook LA, Grant M, Hagerty D (2007) Plasma methylglyoxal and glyoxal are elevated and related to early membrane alteration in young, complication-free patients with Type 1 diabetes. Mol Cell Biochem 305:123–131
Treins C, Giorgetti-Peraldi S, Murdaca J, Van Obberghen E (2001) Regulation of vascular endothelial growth factor expression by advanced glycation end products. J Biol Chem 276:43836–43841
Howes KA, Liu Y, Dunaief JL, Milam A, Frederick JM, Marks A, Baehr W (2004) Receptor for advanced glycation end products and age-related macular degeneration. Invest Ophthalmol Vis Sci 45:3713–3720
Honda S, Farboud B, Hjelmeland LM, Handa JT (2001) Induction of an aging mRNA retinal pigment epithelial cell phenotype by matrix-containing advanced glycation end products in vitro. Invest Ophthalmol Vis Sci 42:2419–2425
Ihnat MA, Thorpe JE, Kamat CD, Szabo C, Green DE, Warnke LA, Lacza Z, Cselenyak A, Ross K, Shakir S, Piconi L, Kaltreider RC, Ceriello A (2007) Reactive oxygen species mediate a cellular ‘memory’ of high glucose stress signalling. Diabetologia 50:1523–1531
Lin T, Walker GB, Kurji K, Fang E, Law G, Prasad SS, Kojic L, Cao S, White V, Cui JZ, Matsubara JA (2013) Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: implications for age-related degenerative diseases of the eye. Cytokine 62:369–381
Ablonczy Z, Dahrouj M, Tang PH, Liu Y, Sambamurti K, Marmorstein AD, Crosson CE (2011) Human retinal pigment epithelium cells as functional models for the RPE in vivo. Invest Ophthalmol Vis Sci 52:8614–8620
Thurman JM, Renner B, Kunchithapautham K, Ferreira VP, Pangburn MK, Ablonczy Z, Tomlinson S, Holers VM, Rohrer B (2009) Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem 284:16939–16947
Niwa H, Takeda A, Wakai M, Miyata T, Yasuda Y, Mitsuma T, Kurokawa K, Sobue G (1998) Accelerated formation of N epsilon-(carboxymethyl) lysine, an advanced glycation end product, by glyoxal and 3-deoxyglucosone in cultured rat sensory neurons. Biochem Biophys Res Commun 248:93–97
Glomb MA, Monnier VM (1995) Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J Biol Chem 270:10017–10026
Shangari N, O’Brien PJ (2004) The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem Pharmacol 68:1433–1442
Bonnefont-Rousselot D (2002) Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care 5:561–568
Cai J, Nelson KC, Wu M, Sternberg P Jr, Jones DP (2000) Oxidative damage and protection of the RPE. Prog Retin Eye Res 19:205–221
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1129–1132
Ookawara T, Kawamura N, Kitagawa Y, Taniguchi N (1992) Site-specific and random fragmentation of Cu,Zn-superoxide dismutase by glycation reaction Implication of reactive oxygen species. J Biol Chem 267:18505–18510
Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC (2000) Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol 2:318–325
Kasper M, Roehlecke C, Witt M, Fehrenbach H, Hofer A, Miyata T, Weigert C, Funk RH, Schleicher ED (2000) Induction of apoptosis by glyoxal in human embryonic lung epithelial cell line L132. Am J Respir Cell Mol Biol 23:485–491
Kniep EM, Roehlecke C, Ozkucur N, Steinberg A, Reber F, Knels L, Funk RH (2006) Inhibition of apoptosis and reduction of intracellular pH decrease in retinal neural cell cultures by a blocker of carbonic anhydrase. Invest Ophthalmol Vis Sci 47:1185–1192
Reber F, Kasper M, Siegner A, Kniep E, Seigel G, Funk RH (2002) Alteration of the intracellular pH and apoptosis induction in a retinal cell line by the AGE-inducing agent glyoxal. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 240: 1022–1032
Li J, Eastman A (1995) Apoptosis in an interleukin-2-dependent cytotoxic T lymphocyte cell line is associated with intracellular acidification. Role of the Na(+)/H(+)-antiport. J Biol Chem 270:3203–3211
Ishaque A, Al-Rubeai M (1998) Use of intracellular pH and annexin-V flow cytometric assays to monitor apoptosis and its suppression by bcl-2 over-expression in hybridoma cell culture. J Immunol Methods 221:43–57
Overbeeke R, Yildirim M, Reutelingsperger CP, Haanen C, Vermes I (1999) Sequential occurrence of mitochondrial and plasma membrane alterations, fluctuations in cellular Ca2+ and pH during initial and later phases of cell death. Apoptosis 4:455–460
Hirpara JL, Clement MV, Pervaiz S (2001) Intracellular acidification triggered by mitochondrial-derived hydrogen peroxide is an effector mechanism for drug-induced apoptosis in tumor cells. J Biol Chem 276:514–521
Wenzel U, Daniel H (2004) Early and late apoptosis events in human transformed and non-transformed colonocytes are independent on intracellular acidification. Cell Physiol Biochem 14:65–76
Witmer AN, Vrensen GFJM, Van Noorden CJF, Schlingemann RO (2003) Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 22:1–29
Behl T, Kotwani A (2015) Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol Res 99:137–148
Lu M, Kuroki M, Amano S, Tolentino M, Keough K, Kim I, Bucala R, Adamis AP (1998) Advanced glycation end products increase retinal vascular endothelial growth factor expression. J Clin Invest 101:1219–1224
Mamputu JC, Renier G (2002) Advanced glycation end products increase, through a protein kinase C-dependent pathway, vascular endothelial growth factor expression in retinal endothelial cells. Inhibitory effect of gliclazide. J Diabetes Complications 16:284–293
Yamagishi S, Yonekura H, Yamamoto Y, Katsuno K, Sato F, Mita I, Ooka H, Satozawa N, Kawakami T, Nomura M, Yamamoto H (1997) Advanced glycation end products-driven angiogenesis in vitro. Induction of the growth and tube formation of human microvascular endothelial cells through autocrine vascular endothelial growth factor. J Biol Chem 272:8723–8730
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (funding of the Excellence Initiative by the German Federal and State Governments, Institutional Strategy, measure “support the best”, to HM and RHWF, MO 1695/4-1 and MO 1695/5-1 to HM). The authors thank T. Schwalm for technical assistance and E.D. Schleicher for kindly providing anti-CML antiserum. We also thank Kathy Eisenhofer for critically reading the manuscript.
Contributors
CR, MV, LK, and RHWF designed the study and were involved in data production, data evaluation, and manuscript writing. AF and HM designed and performed experiments on VEGF165 determination, evaluated the data and were involved in manuscript writing. DG performed caspase 3 Western blotting, evaluated the data and was involved in manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (funding of the Excellence Initiative by the German Federal and State Governments, Institutional Strategy, measure “support the best” to HM and RHWF, MO 1695/4-1 and MO 1695/5-1 to HM).
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Roehlecke, C., Valtink, M., Frenzel, A. et al. Stress responses of human retinal pigment epithelial cells to glyoxal. Graefes Arch Clin Exp Ophthalmol 254, 2361–2372 (2016). https://doi.org/10.1007/s00417-016-3463-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3463-2