Abstract
Purpose
The aim of this study was to investigate biofilm formation on silicone tubes by genetically diverse methicillin-resistant Staphylococcus aureus (MRSA) strains.
Methods
Capacity of biofilm formation on dacryocystorhinostomy silicone tubes was tested on 30 MRSA strains. Identification and methicillin resistance were confirmed by PCR for nuc and mecA genes. Strains were genotypically characterised (SCCmec, agr and spa typing). Biofilm formation was tested in microtiter plate and on silicone tubes.
Results
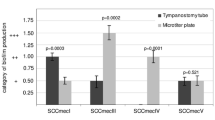
Tested MRSA strains were classified into SCCmec type I (33.3 %), II (3.3 %), III (20.0 %), IV (26.7 %) and V (16.7 %), agr type I (56.7 %), II (36.7 %) and III (6.6 %), and eight spa clonal complexes (CCs). All tested MRSA strains showed ability to form biofilm on microtiter plate. Capacity of biofilm formation on silicone tubes was as follows: 33.3 % of strains belonged to the category of low biofilm producers, and 66.7 % to moderate biofilm producers. There was statistically significant correlation between spa CC and the category of biofilm production on silicone tubes (p = 0.01): CC5 and CC45 with moderate amount of biofilm, and CC8 with low amount of biofilm. A moderate amount of biofilm formed on silicone tubes correlated with agr type II MRSA strains (p = 0.008).
Conclusions
Biofilm formation by MRSA on silicone tubes is highly dependent on genetic characteristics of the strains. Therefore, MRSA genotyping may aid the determination of the possibility of biofilm-related ocular device infections. Genotyping and biofilm quantification may be helpful in determining when decolonisation and cohort isolation are required to prevent device-related infections.

Similar content being viewed by others
References
Ben Simon GJ, Joseph J, Lee S, Schwarcz RM, McCann JD, Goldberg RA (2005) External versus endoscopic dacryocystorhinostomy for acquired nasolacrimal duct obstruction in a tertiary referral center. Ophthalmology 112:1463–1468
Quickert MH, Dryden RM (1970) Probes for intubation in lacrimal drainages. Trans Am Acad Ophtha Otolaryngol 74:431–433
Choung HK, Khwarg SI (2007) Selective non-intubation of a silicone tube in external dacryocystorhinostomy. Acta Ophthalmol Scand 85:329–332
Kim SE, Lee SJ, Lee SY, Yoon JS (2012) Clinical significance of microbial growth on the surfaces of silicone tubes removed from dacryocystorhinostomy patients. Am J Ophthalmol 153:253–257
Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, Jensen BJ, Killgore G, Tenover FC, Kuehnert MJ (2008) Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis 197:1226–1234
Moellering RC (2012) MRSA: the first half century. J Antimicrob Chemother 67:4–11
Gotz F (2002) Staphylococcus and biofilms. Mol Microbiol 43:1367–1378
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193
Bharathi MJ, Ramakrishnan R, Maneksha V, Shivakumar C, Nithya V, Mittal S (2008) Comparative bacteriology of acute and chronic dacryocystitis. Eye 22:953–960
Brakstad OG, Aabakk K, Maeland JA (1992) Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol 30:1654–1660
Bignardi GE, Woodford N, Chapman A, Johnson AP, Speller DC (1996) Detection of the mec-A and phenotypic detection of resistance in Staphylococcus aureus isolates with borderline or low-level methicillin resistance. J Antimicrob Chemother 37:53–63
Boye K, Bartels MD, Andersen IS, Møller JA, Westh H (2007) A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCCmec types I-V. Clin Microbiol Infect 13:725–727
Lina G, Boutite F, Tristan A, Bes M, Etienne J, Vandenesch F (2003) Bacterial competition for human nasal cavity colonization: role of Staphylococcal agr alleles. Appl Environ Microbiol 69:18–23
Harmsen D, Claus H, Witte W, Rothgänger J, Claus H, Turnwald D, Vogel U (2003) Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 41:5442–5448
Stepanović S, Vuković D, Hola V, Di Bonaventura G, Djukić S, Cirković I, Ruzicka F (2007) Quantification of biofilm microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115:891–899
Behlau I, Gilmore MS (2008) Microbial biofilms in ophthalmology and infectious disease. Arch Ophthalmol 126:1572–1581
Zegans ME, Shanks RM, O’Toole GA (2005) Bacterial biofilms and ocular infections. Ocul Surf 3:73–80
Darouiche RO (2004) Treatment of infections associated with surgical implants. N Engl J Med 350:1422–1429
Coden DJ, Hornblass A, Haas BD (1993) Clinical bacteriology of dacryocystitis in adults. Ophthal Plast Reconstr Surg 9:125–131
Mandal R, Banerjee AR, Biswas MC, Mondal A, Kundu PK, Sasmal NK (2008) Clinicobacteriological study of chronic dacryocystitis in adults. J Indian Med Assoc 106:296–298
Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL (2005) The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5:751–762
Reem RE, Van Balen J, Hoet AE, Cebulla CM (2014) Screening and characterization of Staphylococcus aureus from ophthalmology clinic surfaces: a proposed surveillance tool. Am J Ophthalmol 157:781–787
Ćirković I, Đukić S, Carević B, Mazić N, Mioljević V, Stepanović S (2014) Methicillin-resistant Staphylococcus aureus nasal carriage among hospitalized patients and healthcare workers in the Clinical Center of Serbia. Arch Biol Sci 66:87–92
O’Gara JP (2007) ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett 270:179–188
Cassat JE, Lee CY, Smeltzer MS (2007) Investigation of biofilm formation in clinical isolates of Staphylococcus aureus. Methods Mol Biol 391:127–144
Cha JO, Yoo JI, Yoo JS, Chung HS, Park SH, Kim HS, Lee YS, Chung GT (2013) Investigation of biofilm formation and its association with the molecular and clinical characteristics of methicillin-resistant Staphylococcus aureus. Osong Public Health Res Perspect 4:225–232
Smith K, Perez A, Ramage G, Lappin D, Gemmell CG, Lang S (2008) Biofilm formation by Scottish clinical isolates of Staphylococcus aureus. J Med Microbiol 57:1018–1023
Archer NK, Mazaitis MJ, Costerton W, Leid JG, Powers ME, Shirtliff ME (2011) Staphylococcus aureus biofilms: properties, regulation and roles in human disease. Virulence 2:445–459
Atshan SS, Shamsudin MN, Lung LT, Sekawi Z, Ghaznavi-Rad E, Pei CP (2012) Comparative characterisation of genotypically different clones of MRSA in the production of biofilms. J Biomed Biotechnol 2012:417247. doi:10.1155/2012/417247
Manago K, Nishi J, Wakimoto N, Miyanohara H, Sarantuya J, Tokuda K, Iwashita M, Yamamoto K, Yoshinaga M, Maruyama I, Kawano Y (2006) Biofilm formation by and accessory gene regulator typing of methicillin-resistant Staphylococcus aureus strains recovered from patients with nosocomial infections. Infect Control Hosp Epidemiol 27:188–190
Wilcox MH, Hall J, Pike H, Templeton PA, Fawley WN, Parnell P, Verity P (2003) Use of perioperative mupirocin to prevent methicillin-resistant Staphylococcus aureus (MRSA) orthopaedic surgical site infections. J Hosp Infect 54:196–201
Muto CA, Jernigan JA, Ostrowsky BE, Richet HM, Jarvis WR, Boyce JM, Farr BM, SHEA (2003) SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Cont Hosp Epidemiol 24:362–386
Acknowledgments
Research reported in this publication was supported by the Ministry of Education, Science and Technological Development, Republic of Serbia (project no. ON175039).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ćirković, I., Knežević, M., Božić, D.D. et al. Methicillin-resistant Staphylococcus aureus biofilm formation on dacryocystorhinostomy silicone tubes depends on the genetic lineage. Graefes Arch Clin Exp Ophthalmol 253, 77–82 (2015). https://doi.org/10.1007/s00417-014-2786-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2786-0